2 - ZOAK FKIT
2 - ZOAK FKIT
2 - ZOAK FKIT
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
IV• CO 2H 2CO 3soli• SiO 2H 4SiO 4soli• GeO 2(H 4GeO 4) soli• SnO 2←(Sn(OH) 4) soli• PbO 2? soli
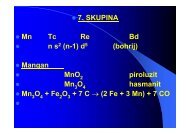
• CO 2Dobivanje• Lab.: CaCO 3+ 2 H + →Ca 2+ + CO 2+ H 2O• Ind: CaCO 3⇔ CaO +CO 2• C 6H 12O 6→ 2 C 2H 5OH + 2 CO 2• K 2CO 3+ CO 2+ H 2O ⇔ 2 KHCO 3•kuhanje•• CO 2+ 2 Mg → 2 MgO + C
• CO 2+H 2O ⇔H 2CO 3⇔ H + + HCO 3- ⇔ 2 H + + CO 32-• K 1 =[ H + ][HCO 3-] ≈• [ H 2CO3]• K 1 =[ H + ][HCO 3-] ≈• [ CO 2]• K 2 =[ H + ][HCO 3-] ≈• [ HCO 3-]1 ⋅10 -4 mol/L3 ⋅10 -7 mol/L5 ⋅10 -11 mol/L
• CO 32-+ H 2O ⇔ HCO 3-+ OH -• HCO 3-+ H 2O ⇔ H 2CO 3+ OH -•grijanje• 2 HCO 3-⇔ CO 32-+ CO 2+ H 2O• HCO 3- + OH - → CO 32-+ H 2O• Soli Na 2CO 3, NaHCO 3
• NaCl → Na + + Cl -• Solvayev postupak• NH 3+ H 2O→ NH 4+ + OH -• CO 2+ OH - → HCO 3-• Na + + HCO 3→ NaHCO 3(s)• 2 NaHCO 3→ Na 2CO 3+ CO 2+ H 2O• CaCO 3→ CaO + CO 2• CaO + H 2O→ Ca 2+ + 2 OH -• 2 NH 4++ 2 Cl - + Ca 2+ + 2 OH - → 2 NH 3+ 2H 2O + Ca 2+ + 2 Cl -
• K + + OH - + CO 2→ KHCO 3• 2 KHCO 3→ K 2CO 3+ CO 2+ H 2O• C + 2 S → CS 2• 2 CS 2+ 5 O 2→ 2 CO + 4 SO 2• CS 2+ 3 Cl 2→ CCl 4+ S 2Cl 2
• IV Si• SiO 2+ 4 HF→SiF 4+ 2 H 2O• SiF 4+ 2 HF→ H 2[SiF 6] [H 3O + ] 2[SiF2-6]• SiO 2+ 2 NaOH (l) → Na 2SiO 3+ H 2O• SiCl 4+ H 2O→H 4SiO 4+ 4 H + + 4 Cl -• 2 H 4SiO 4→ H 6Si 2O 7+H 2O
[Si 3O 9] 6- (H 2SiO 3) 3[ Si 6O 18] 12- (H 2SiO 3) 6
(H 6Si 4O 11) n[Si 4O 11] 6-
• (H 2Si 2O 5)n [Si 2O 5] 2-kaolin Al 2(OH) 4Si 2O 5talk Mg 3(OH) 2Si 4O 10muskovit KAl 2(OH) 2Si 3AlO 10
-Y H2O• x H 4SiO 4→ (H 2SiO 3) xx : y = 1: 1• (H 6Si 4O 11) nx : y = 4: 5• (H 2Si 2O 5) nx : y = 2: 3• (SiO 2) nx : y = 1: 2
• SiO 2+ Na 2CO 3→ Na 2SiO 3+ CO 2• 2 SiO 2+ Na 2CO 3→ Na 2Si 2O 5+ CO 2• Na + + SiO 32-+ H + + Cl - → H 2SiO 3+ 2 Na + + 2 Cl -• SiO 2+ 2 C + 2 Cl 2→ SiCl 4+ 2 CO• RSiCl 3, R 2SiCl 2, R 3SiCl• R 2SiCl 2+ 2 H 2O→R 2Si(OH) 2+ 2 HCl• n (R 2Si(OH) 2→ (R 2SiO) n+ n H 2O
• IV Sn• Sn + 2 Cl 2→ SnCl 4•H2SO4• SnCl 4+ 2 H 2O → SnO 2+ 4 HCl• SnCl 4« spiritus fumans Libavi»• SnCl 4+ 2 HCl → H 2[SnCl 6]H 2[SnCl 6] ⋅2 H 2O• SnCl 4+ 2 NH 4Cl → (NH 4) 2SnCl 6• «pinkova sol»•• Sn + 2 S → SnS 2«musivno zlato»
• IV Pb•200ºC• PbO 2→ PbO + ½ O 2• PbO 2+ 4 H + + 2 e - → Pb 2+ + 2 H 2O E 0 = 1.46V• PbO 2+ OH - + 2 H 2O → [Pb(OH) 6] 2-• konc
• Dob. PbO 2• Pb(OH) 3-+ ClO - → PbO 2+ Cl - + OH - + H 2O• Akumulator• - Pb + HSO 4-⇔ PbSO 4(s) + H + + 2 e -• - PbO 2+ 3 H ++ HSO 4+ 2 e - ⇔ PbSO 4(s) + 2 H 2O•• Minij• Pb 3O 4Pb 2PbO 4•500°C• Dob: 3 PbO + ½ O 2→ Pb 3O 4• Pb 2PbO 4+ 4 H + → 2 Pb 2+ + H 4PbO 4• ⎢• ⎩ PbO 2+ 2 H 2O
13 SKUPINA ns 2 np 1B Al Ga In Tl• E i/eV 8.3 6 6 5.8 6.1• χ 2.0 1.5 1.6 1.7 1.8• E 0 III / I•/V -1.66 -0.53 -0.34 +0.7• Raste energija hidratacije
ELEMENTARNE TVARI• B•kernitNa 2B 4O 7 · 4 H 2O• Na 2[B 4O 5(OH) 4] · 8 H 2OBoraks• Stara formula : Na 2B 4O 7 · 10 H 2O• Dob:H+ grijanje• Na 2B 4O 7→ H 3BO 3→ B 2O 3•1900°C• B 2O 3+ 3 Mg → 2 B + 3 MgO
•Ta• 2 BBr 3+ H 2 → 2 B + 6 HBr•1200°C• 2 Cu 2O + 2 B → 6 Cu + B 2O 3
• Al• Boksit: AlO(OH) ⎡ bemit• ⎣dijaspor• Al(OH) 3hidrargilit• Dob Al: dobivanje čistog Al 2O 3• Elektroliza taline• Bayerov postupak•160 °C, 3.5 MPa• Al(OH) 3+ OH - ⇔ [Al(OH) 4] -•Razr• SiO 2+ 2 NaOH + 2 Al(OH) 3→Na 2Al 2SiO 6+ 4 H 2O•1200°C• 2 Al(OH) 3→Al 2O 3+ 3 H 2O
• Crveni mulj: sastav (suhe tvari)• w (Fe 2O 3) do 0.6• w (Al 2O 3) do 0.2• w (TiO 2) do 0.1• Ostatak Na 2O i SiO 2• Suhi postupak:• 1873 Le Chatelier• Al 2O 3+ Na 2CO 3→2 Na AlO 2+ CO 2• NaAlO 2+ 2 H 2O→ Na + + [Al(OH) 4] -• [Al(OH) 4] - + CO 2→Al(OH) 3+ HCO 3-• NaFeO 2+ 2 H 2O →Fe(OH) 3+ Na + + OH -
• Redukcija glinice• Al 2O 3t t≈ 2000 °C• Na 3AlF 6t t≈1000 °C• K: 2 Al 2O 3+ 6 e - → 2 Al + 2 AlO 33-• A: 2 AlO 33-+ 3 C → 3 CO + 6 e - + Al 2O 3• Al 2O 3+ 3 C → 2 Al + 3 CO
• Kem. Svojstva• 2 Al + 3 Hg 2+ → 2 Al 3+ + 3 Hg• Al + Hg → Al(Hg)• 2 Al(Hg) + 3/2 O 2→ Al 2O 3+ 2 Hg• 2 Al(Hg) + 6 H 2O→ 2 Al(OH) 3+ 3 H 2+ 2 Hg• Al + 3 H + + 6 H 2O →[Al(H 2O) 6] 3+ + 3/2 H 2• Al + 3 H 2O + OH - →[Al(H 2O) 4] - + 3/2 H 2• 2 Al + 3/2 O 2→ Al 2O 3+ hν ∆ rH= -1670 kJ/mol• Cr 2O 3+ 2 Al →2 Cr + Al 2O 3∆ rH < 0• Fe 2O 3+ 2 Al →2 Fe + Al 2O 3∆ rH < 0
Pregled reakcija• 2 M + 3 X 2→ 2 MX 3• X = halogenosim TlX• 3 M + 3 O 2→2 M 2O 3osim TlO 2• 2 M + 3 S → M 2S 3Tl 2S kod viših temp.• 2 M + N 2→ 2MN samo B, Al kod viših t.• 2 M + 6 H + → 2 M 3+ + 3 H 2Al, Ga, In, Tl →Tl +• M+ OH - + 3 H 2O →[M (OH) 4] - + 3/2 H 2, Al, Ga
SPOJEVI:• I• IIITlB, Al, Ga, In• Negativni stupanj oksidacije - boridi• TiB 2, ZrB 2← t t= 3000°C