Zborník príspevkov z vedeckej konferencie - Department of ...
Zborník príspevkov z vedeckej konferencie - Department of ... Zborník príspevkov z vedeckej konferencie - Department of ...
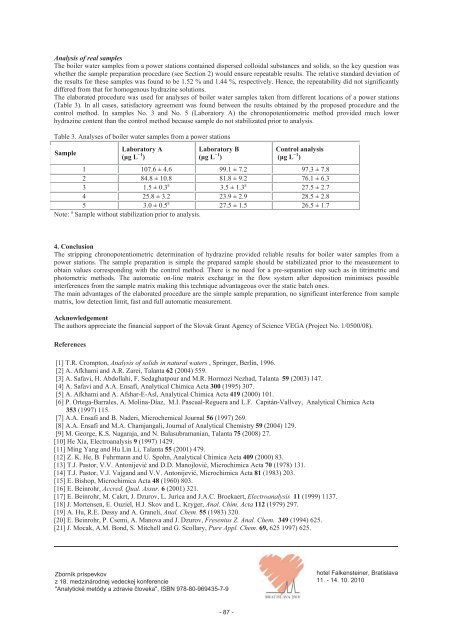
Analysis of real samples The boiler water samples from a power stations contained dispersed colloidal substances and solids, so the key question was whether the sample preparation procedure (see Section 2) would ensure repeatable results. The relative standard deviation of the results for these samples was found to be 1.52 % and 1.44 %, respectively. Hence, the repeatability did not significantly differed from that for homogenous hydrazine solutions. The elaborated procedure was used for analyses of boiler water samples taken from different locations of a power stations (Table 3). In all cases, satisfactory agreement was found between the results obtained by the proposed procedure and the control method. In samples No. 3 and No. 5 (Laboratory A) the chronopotentiometric method provided much lower hydrazine content than the control method because sample do not stabilizated prior to analysis. Table 3. Analyses of boiler water samples from a power stations Sample Laboratory A (g L 1 ) Laboratory B (g L 1 ) Control analysis (g L 1 ) 1 107.6 ± 4.6 99.1 ± 7.2 97.3 ± 7.8 2 84.8 ± 10.8 81.8 ± 9.2 76.1 ± 6.3 3 1.5 ± 0.3 a 3.5 ± 1.3 a 27.5 ± 2.7 4 25.8 ± 3.2 23.9 ± 2.9 28.5 ± 2.8 5 3.0 ± 0.5 a 27.5 ± 1.5 26.5 ± 1.7 Note: a Sample without stabilization prior to analysis. 4. Conclusion The stripping chronopotentiometric determination of hydrazine provided reliable results for boiler water samples from a power stations. The sample preparation is simple the prepared sample should be stabilizated prior to the measurement to obtain values corresponding with the control method. There is no need for a pre-separation step such as in titrimetric and photometric methods. The automatic on-line matrix exchange in the flow system after deposition minimises possible interferences from the sample matrix making this technique advantageous over the static batch ones. The main advantages of the elaborated procedure are the simple sample preparation, no significant interference from sample matrix, low detection limit, fast and full automatic measurement. Acknowledgement The authors appreciate the financial support of the Slovak Grant Agency of Science VEGA (Project No. 1/0500/08). References [1] T.R. Crompton, Analysis of solids in natural waters , Springer, Berlin, 1996. [2] A. Afkhami and A.R. Zarei, Talanta 62 (2004) 559. [3] A. Safavi, H. Abdollahi, F. Sedaghatpour and M.R. Hormozi Nezhad, Talanta 59 (2003) 147. [4] A. Safavi and A.A. Ensafi, Analytical Chimica Acta 300 (1995) 307. [5] A. Afkhami and A. Afshar-E-Asl, Analytical Chimica Acta 419 (2000) 101. [6] P. Ortega-Barrales, A. Molina-Díaz, M.I. Pascual-Reguera and L.F. Capitán-Vallvey, Analytical Chimica Acta 353 (1997) 115. [7] A.A. Ensafi and B. Naderi, Microchemical Journal 56 (1997) 269. [8] A.A. Ensafi and M.A. Chamjangali, Journal of Analytical Chemistry 59 (2004) 129. [9] M. George, K.S. Nagaraja, and N. Balasubramanian, Talanta 75 (2008) 27. [10] He Xia, Electroanalysis 9 (1997) 1429. [11] Ming Yang and Hu Lin Li, Talanta 55 (2001) 479. [12] Z. K. He, B. Fuhrmann and U. Spohn, Analytical Chimica Acta 409 (2000) 83. [13] T.J. Pastor, V.V. Antonijevi and D.D. Manojlovi, Microchimica Acta 70 (1978) 131. [14] T.J. Pastor, V.J. Vajgand and V.V. Antonijevi, Microchimica Acta 81 (1983) 203. [15] E. Bishop, Microchimica Acta 48 (1960) 803. [16] E. Beinrohr, Accred. Qual. Assur. 6 (2001) 321. [17] E. Beinrohr, M. Cakrt, J. Dzurov, L. Jurica and J.A.C. Broekaert, Electroanalysis 11 (1999) 1137. [18] J. Mortensen, E. Ouziel, H.J. Skov and L. Kryger, Anal. Chim. Acta 112 (1979) 297. [19] A. Hu, R.E. Dessy and A. Graneli, Anal. Chem. 55 (1983) 320. [20] E. Beinrohr, P. Csemi, A. Manova and J. Dzurov, Fresenius Z. Anal. Chem. 349 (1994) 625. [21] J. Mocak, A.M. Bond, S. Mitchell and G. Scollary, Pure Appl. Chem. 69, 625 1997) 625. Zborník príspevkov z 18. medzinárodnej vedeckej konferencie "Analytické metódy a zdravie loveka", ISBN 978-80-969435-7-9 - 87 - hotel Falkensteiner, Bratislava 11. - 14. 10. 2010
POSSIBLE DIAGNOSIS OF OVARIAN CANCER BY SYNCHRONOUS FLUORESCENCE SPECTRA OF URINE 1 MILAN ZVARÍK*, 1 LIBUŠA ŠIKUROVÁ and 2 UBA HUNÁKOVÁ 1 Division of Biomedical Physics, FMPHI Comenius University, Mlynská dolina, 842 48, Bratislava, Slovakia 2 Cancer Research Institute Slovak Academy of Sciences, Vlárska 7, 833 91 Bratislava, Slovakia zvarikmilan@gmail.com Introduction Urine is a complex biological fluid containing a range of chemical compounds produced by the body, some of which are fluorescent. The concentration of fluorophores in urine is influenced by many factors including body metabolism, dietary intake, age, and various diseases [1] what might be used for quick diagnostic test. In this contribution, we have examined synchronous fluorescence spectra of urine for possible detection of ovarian cancer. Material and methods Samples of urine were obtained from healthy (control) and experimental BALB/c athymic nude mice. Human ovarian cancer cells SKOV-3 were implanted into the peritoneal cavities of experimental mouse. Urine samples were centrifuged at 3000 rpm for 10 min at room temperature (22 ± 1ºC) and supernatants were used undiluted or diluted with deionized water (1:1 - 1:1024) for spectral analysis. The fluorescence spectra were obtained on a LS45 (PerkinElmer) luminescence spectrometer at room temperature (22 ± 1ºC). Synchronous fluorescence spectra (SFS) were collected by simultaneously scanning the excitation and emission monochromator in the excitation wavelength range 250 - 550 nm, with constant wavelength differences between them. Spectra were recorded for interval from 20 to 100 nm, in steps of 10 nm. Concentration matrices were made of synchronous fluorescence spectra of urine with various dilutions. Fluorescence excitation/emission matrices (EEMs) were acquired of excitation wavelength range from 250 to 550 nm in intervals of 20 nm, where emission spectra were taken in intervals of 250 - 650 nm. Obtained spectral data were processed using FL WinLab software. Results and discussion The excitation/emission matrices (EEMs) of diluted mouse urine for both control and experimental animals showed three fluorescence emission peaks at around 390, 420 and 520 nm (Fig 1). In the control sample the first fluorescence emission peak at around 390 nm was created by changing the excitation wavelength from 250 nm to 290 nm and it reached the maximal fluorescent intensity with excitation at 290 nm (Fig 2a). The second emission fluorescence peak of control sample was shifted from 410 nm to 450 nm with increasing the excitation wavelength from 310 nm to 390 nm and it completely disappeared above 390 nm excitation (Fig 2b). The third (weak) fluorescence peak occurred in the area of 520 nm and reached its maximum by excitations at 370 nm and 450 nm (Fig. 2b). The experimental sample as well as control one showed the first fluorescence peak at 390 nm, while its intensity was lower than in the control (- 44 %) (Fig 2a). The second fluorescence peak of the cancer sample was of the same nature as in the control sample, but the intensity of fluorescence was higher (Fig. 2b). The third fluorescence peak at 520 nm reached its maximum by excitation at 370 nm and 450 nm, too, however the intensity of fluorescence for experimental mouse urine was much higher than it was in the control group (ex 370 -55%, ex 450 -70%) (Fig. 2b). Zborník príspevkov z 18. medzinárodnej vedeckej konferencie "Analytické metódy a zdravie loveka", ISBN 978-80-969435-7-9 - 88 - hotel Falkensteiner, Bratislava 11. - 14. 10. 2010
- Page 44 and 45: Tabuka 4 Bioakumulovaný Zn jednotl
- Page 46 and 47: Úbytok kovu v % 100 80 60 40 20 0
- Page 48 and 49: DEVELOPMENT OF THE SOLID SAMPLING E
- Page 50 and 51: 3. Results and discussion 3.1. Meth
- Page 52 and 53: different atomization signal profil
- Page 54 and 55: Financial support from the Scientif
- Page 56 and 57: Zhydrolyzované ftaláty boli deriv
- Page 58 and 59: UVONENIE PRCHAVÝCH ORGANICKÝCH ZL
- Page 60 and 61: a 3B) v headspace bunkách CALU-1 v
- Page 62 and 63: koncentrácia niekokých alkánov a
- Page 64 and 65: Obrázok 4: A a B: Porovnanie konce
- Page 66 and 67: TEPLOTNE PROGRAMOVANÉ PLYNOVO CHRO
- Page 68 and 69: metyl x-metyl-y-oát OV 1 I P s OV
- Page 70 and 71: metyl x-metyl-y-oát OV 1 I P s OV
- Page 72 and 73: Fig. 1. Chromatogram GC separácie
- Page 74 and 75: OV 1 Obr. 3. Závislos homomorfnýc
- Page 76 and 77: Záver Namerali sa teplotne-program
- Page 78 and 79: modifier solutions for ensure homog
- Page 80 and 81: applied to attain a stabilization o
- Page 82 and 83: against aqueous standards with a pr
- Page 84 and 85: Ludrová (6). Maslo sa vyrobilo zmi
- Page 86 and 87: Tabuka 2. Zloženie mastných kysel
- Page 88 and 89: Tabuka 3. Obsah jednotlivých izom
- Page 90 and 91: [19] J. Blaško, R. Kubinec, I. Ost
- Page 92 and 93: 2. Experimental Instrumentation Flo
- Page 96 and 97: a) b) 2 2 1 1 3 3 3 3 Fig. 1 The ex
- Page 98 and 99: Conclusion remarks The identificati
- Page 100 and 101: screening, possible interactions am
- Page 102 and 103: The design consists of a factorial
- Page 104 and 105: elative area 15 10 5 -1,68 -1,00 0,
- Page 106 and 107: It can be seen that the programms d
- Page 108 and 109: nevodíkovými atómami izotropne s
- Page 110 and 111: O22—C6—C7 109.57 (15) C10—C11
- Page 112 and 113: [8] S. Teklu, L.L. Gundersen, T. La
- Page 114 and 115: iónom kovu môže by ovplyvnená a
- Page 116 and 117: V alšej asti práce sme študovali
- Page 118 and 119: vzorky, pomer hmotností vzorky a s
- Page 120 and 121: literatúre nenašli informácie. M
- Page 122 and 123: Záver Pri úprave vzorky pôdy met
- Page 124 and 125: galactose-4-epimerase [6, 7]. First
- Page 126 and 127: Abundance Abundance Abundance 1x10
- Page 128 and 129: Galactitol [mmol/mol creatinine] 60
- Page 130 and 131: References [1] J.T.R. Clarke, Clini
- Page 132 and 133: structural information of the analy
- Page 134 and 135: (x10,000,000) 1.5 1:TIC (1.00) 1.4
- Page 136 and 137: 1 st fraction 1.5 1.0 0.5 (x10,000,
- Page 138 and 139: 1 st fraction 1.5 1.0 0.5 (x10,000,
- Page 140 and 141: RAPID LIQUID CHROMATOGRAPHY-MASS SP
- Page 142 and 143: the LC-ESI-IT-TOF MS analyzer. HPLC
Analysis <strong>of</strong> real samples<br />
The boiler water samples from a power stations contained dispersed colloidal substances and solids, so the key question was<br />
whether the sample preparation procedure (see Section 2) would ensure repeatable results. The relative standard deviation <strong>of</strong><br />
the results for these samples was found to be 1.52 % and 1.44 %, respectively. Hence, the repeatability did not significantly<br />
differed from that for homogenous hydrazine solutions.<br />
The elaborated procedure was used for analyses <strong>of</strong> boiler water samples taken from different locations <strong>of</strong> a power stations<br />
(Table 3). In all cases, satisfactory agreement was found between the results obtained by the proposed procedure and the<br />
control method. In samples No. 3 and No. 5 (Laboratory A) the chronopotentiometric method provided much lower<br />
hydrazine content than the control method because sample do not stabilizated prior to analysis.<br />
Table 3. Analyses <strong>of</strong> boiler water samples from a power stations<br />
Sample<br />
Laboratory A<br />
(g L 1 )<br />
Laboratory B<br />
(g L 1 )<br />
Control analysis<br />
(g L 1 )<br />
1 107.6 ± 4.6 99.1 ± 7.2 97.3 ± 7.8<br />
2 84.8 ± 10.8 81.8 ± 9.2 76.1 ± 6.3<br />
3 1.5 ± 0.3 a<br />
3.5 ± 1.3 a 27.5 ± 2.7<br />
4 25.8 ± 3.2 23.9 ± 2.9 28.5 ± 2.8<br />
5 3.0 ± 0.5 a<br />
27.5 ± 1.5 26.5 ± 1.7<br />
Note: a Sample without stabilization prior to analysis.<br />
4. Conclusion<br />
The stripping chronopotentiometric determination <strong>of</strong> hydrazine provided reliable results for boiler water samples from a<br />
power stations. The sample preparation is simple the prepared sample should be stabilizated prior to the measurement to<br />
obtain values corresponding with the control method. There is no need for a pre-separation step such as in titrimetric and<br />
photometric methods. The automatic on-line matrix exchange in the flow system after deposition minimises possible<br />
interferences from the sample matrix making this technique advantageous over the static batch ones.<br />
The main advantages <strong>of</strong> the elaborated procedure are the simple sample preparation, no significant interference from sample<br />
matrix, low detection limit, fast and full automatic measurement.<br />
Acknowledgement<br />
The authors appreciate the financial support <strong>of</strong> the Slovak Grant Agency <strong>of</strong> Science VEGA (Project No. 1/0500/08).<br />
References<br />
[1] T.R. Crompton, Analysis <strong>of</strong> solids in natural waters , Springer, Berlin, 1996.<br />
[2] A. Afkhami and A.R. Zarei, Talanta 62 (2004) 559.<br />
[3] A. Safavi, H. Abdollahi, F. Sedaghatpour and M.R. Hormozi Nezhad, Talanta 59 (2003) 147.<br />
[4] A. Safavi and A.A. Ensafi, Analytical Chimica Acta 300 (1995) 307.<br />
[5] A. Afkhami and A. Afshar-E-Asl, Analytical Chimica Acta 419 (2000) 101.<br />
[6] P. Ortega-Barrales, A. Molina-Díaz, M.I. Pascual-Reguera and L.F. Capitán-Vallvey, Analytical Chimica Acta<br />
353 (1997) 115.<br />
[7] A.A. Ensafi and B. Naderi, Microchemical Journal 56 (1997) 269.<br />
[8] A.A. Ensafi and M.A. Chamjangali, Journal <strong>of</strong> Analytical Chemistry 59 (2004) 129.<br />
[9] M. George, K.S. Nagaraja, and N. Balasubramanian, Talanta 75 (2008) 27.<br />
[10] He Xia, Electroanalysis 9 (1997) 1429.<br />
[11] Ming Yang and Hu Lin Li, Talanta 55 (2001) 479.<br />
[12] Z. K. He, B. Fuhrmann and U. Spohn, Analytical Chimica Acta 409 (2000) 83.<br />
[13] T.J. Pastor, V.V. Antonijevi and D.D. Manojlovi, Microchimica Acta 70 (1978) 131.<br />
[14] T.J. Pastor, V.J. Vajgand and V.V. Antonijevi, Microchimica Acta 81 (1983) 203.<br />
[15] E. Bishop, Microchimica Acta 48 (1960) 803.<br />
[16] E. Beinrohr, Accred. Qual. Assur. 6 (2001) 321.<br />
[17] E. Beinrohr, M. Cakrt, J. Dzurov, L. Jurica and J.A.C. Broekaert, Electroanalysis 11 (1999) 1137.<br />
[18] J. Mortensen, E. Ouziel, H.J. Skov and L. Kryger, Anal. Chim. Acta 112 (1979) 297.<br />
[19] A. Hu, R.E. Dessy and A. Graneli, Anal. Chem. 55 (1983) 320.<br />
[20] E. Beinrohr, P. Csemi, A. Manova and J. Dzurov, Fresenius Z. Anal. Chem. 349 (1994) 625.<br />
[21] J. Mocak, A.M. Bond, S. Mitchell and G. Scollary, Pure Appl. Chem. 69, 625 1997) 625.<br />
<strong>Zborník</strong> <strong>príspevkov</strong><br />
z 18. medzinárodnej <strong>vedeckej</strong> <strong>konferencie</strong><br />
"Analytické metódy a zdravie loveka", ISBN 978-80-969435-7-9<br />
- 87 -<br />
hotel Falkensteiner, Bratislava<br />
11. - 14. 10. 2010