Syllabus PAOKC-cursus Klinische Chemie en ... - NVKC
Syllabus PAOKC-cursus Klinische Chemie en ... - NVKC
Syllabus PAOKC-cursus Klinische Chemie en ... - NVKC
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1570 B. N. Bouma and J. C. M. Meijers<br />
32<br />
prev<strong>en</strong>ts TAFIa formation [35]. On the other hand, TAFIa has<br />
be<strong>en</strong> shown to att<strong>en</strong>uate fibrinolysis by inhibiting plasmin<br />
directly [8,26].<br />
The physiological importance of plasmin as a regulator of<br />
TAFIa is not clear to date. Normally, thrombin g<strong>en</strong>eration will<br />
precede plasmin formation, and the role of plasmin-mediated<br />
TAFI activation, or prev<strong>en</strong>tion thereof, during clot lysis may be<br />
relatively limited. However, TAFI fragm<strong>en</strong>ts resembling those<br />
g<strong>en</strong>erated by plasmin, such as the 44.3-kDa fragm<strong>en</strong>t, have be<strong>en</strong><br />
observed during t-PA-mediated lysis of thrombin-induced clots,<br />
and the 44.3-kDa fragm<strong>en</strong>t was observed in plasmas of pati<strong>en</strong>ts<br />
treated with t-PA after myocardial infarction, but not in plasmas<br />
of untreated pati<strong>en</strong>ts [35]. Decreased levels of functional TAFI<br />
might <strong>en</strong>hance the effect of increased fibrinolysis induced by t-<br />
PA administration. Decreased levels of functional TAFI were<br />
detected in plasmas of pati<strong>en</strong>ts with acute promyelocytic leukemia<br />
[36], whereas antig<strong>en</strong> levels were normal. The reduction in<br />
TAFI was most likely caused by the action of plasmin on TAFI,<br />
because in vitro experim<strong>en</strong>ts revealed that plasmin slightly<br />
reduced TAFI antig<strong>en</strong> levels but severely reduced TAFIa activity.<br />
The acquired functional defici<strong>en</strong>cy in acute promyelocytic<br />
leukemia may contribute to the severity of the hemorrhagic<br />
diathesis because of the impaired capacity of the coagulation<br />
system to protect the fibrin clot from fibrinolysis [36].<br />
A role for the intrinsic coagulation system in the activation<br />
of thrombin-activatable fibrinolysis inhibitor<br />
The activation of TAFI by thrombin implies that the coagulation<br />
system plays a role in the regulation of fibrinolysis and that any<br />
disturbance in the g<strong>en</strong>eration of thrombin will result in an<br />
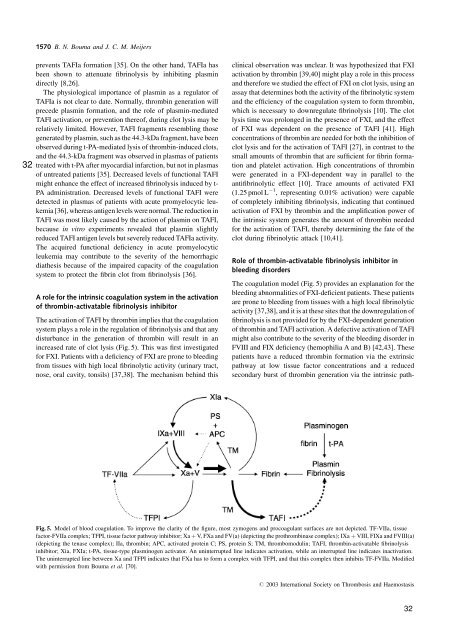
increased rate of clot lysis (Fig. 5). This was first investigated<br />
for FXI. Pati<strong>en</strong>ts with a defici<strong>en</strong>cy of FXI are prone to bleeding<br />
from tissues with high local fibrinolytic activity (urinary tract,<br />
nose, oral cavity, tonsils) [37,38]. The mechanism behind this<br />
clinical observation was unclear. It was hypothesized that FXI<br />
activation by thrombin [39,40] might play a role in this process<br />
and therefore we studied the effect of FXI on clot lysis, using an<br />
assay that determines both the activity of the fibrinolytic system<br />
and the effici<strong>en</strong>cy of the coagulation system to form thrombin,<br />
which is necessary to downregulate fibrinolysis [10]. The clot<br />
lysis time was prolonged in the pres<strong>en</strong>ce of FXI, and the effect<br />
of FXI was dep<strong>en</strong>d<strong>en</strong>t on the pres<strong>en</strong>ce of TAFI [41]. High<br />
conc<strong>en</strong>trations of thrombin are needed for both the inhibition of<br />
clot lysis and for the activation of TAFI [27], in contrast to the<br />
small amounts of thrombin that are suffici<strong>en</strong>t for fibrin formation<br />
and platelet activation. High conc<strong>en</strong>trations of thrombin<br />
were g<strong>en</strong>erated in a FXI-dep<strong>en</strong>d<strong>en</strong>t way in parallel to the<br />
antifibrinolytic effect [10]. Trace amounts of activated FXI<br />
(1.25 pmol L 1 , repres<strong>en</strong>ting 0.01% activation) were capable<br />
of completely inhibiting fibrinolysis, indicating that continued<br />
activation of FXI by thrombin and the amplification power of<br />
the intrinsic system g<strong>en</strong>erates the amount of thrombin needed<br />
for the activation of TAFI, thereby determining the fate of the<br />
clot during fibrinolytic attack [10,41].<br />
Role of thrombin-activatable fibrinolysis inhibitor in<br />
bleeding disorders<br />
The coagulation model (Fig. 5) provides an explanation for the<br />
bleeding abnormalities of FXI-defici<strong>en</strong>t pati<strong>en</strong>ts. These pati<strong>en</strong>ts<br />
are prone to bleeding from tissues with a high local fibrinolytic<br />
activity [37,38], and it is at these sites that the downregulation of<br />
fibrinolysis is not provided for by the FXI-dep<strong>en</strong>d<strong>en</strong>t g<strong>en</strong>eration<br />
of thrombin and TAFI activation. A defective activation of TAFI<br />
might also contribute to the severity of the bleeding disorder in<br />
FVIII and FIX defici<strong>en</strong>cy (hemophilia A and B) [42,43]. These<br />
pati<strong>en</strong>ts have a reduced thrombin formation via the extrinsic<br />
pathway at low tissue factor conc<strong>en</strong>trations and a reduced<br />
secondary burst of thrombin g<strong>en</strong>eration via the intrinsic path-<br />
Fig. 5. Model of blood coagulation. To improve the clarity of the figure, most zymog<strong>en</strong>s and procoagulant surfaces are not depicted. TF-VIIa, tissue<br />
factor-FVIIa complex; TFPI, tissue factor pathway inhibitor; Xa þ V, FXa and FV(a) (depicting the prothrombinase complex); IXa þ VIII, FIXa and FVIII(a)<br />
(depicting the t<strong>en</strong>ase complex); IIa, thrombin; APC, activated protein C; PS, protein S; TM, thrombomodulin; TAFI, thrombin-activatable fibrinolysis<br />
inhibitor; Xia, FXIa; t-PA, tissue-type plasminog<strong>en</strong> activator. An uninterrupted line indicates activation, while an interrupted line indicates inactivation.<br />
The uninterrupted line betwe<strong>en</strong> Xa and TFPI indicates that FXa has to form a complex with TFPI, and that this complex th<strong>en</strong> inhibits TF-FVIIa. Modified<br />
with permission from Bouma et al. [70].<br />
# 2003 International Society on Thrombosis and Haemostasis<br />
32