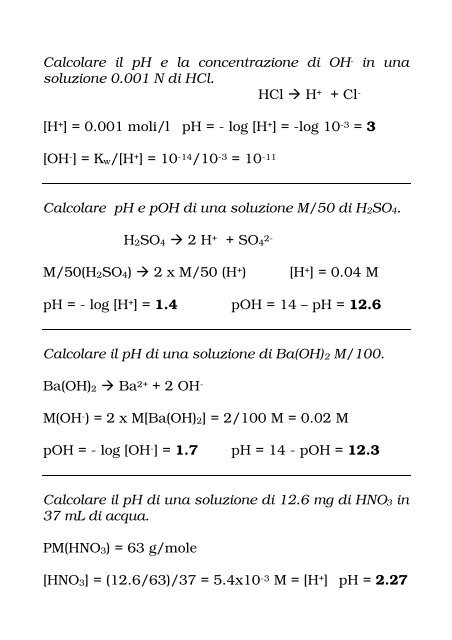
Calcolare il pH e la concentrazione di OH- in una soluzione 0.001 N ...
Calcolare il pH e la concentrazione di OH- in una soluzione 0.001 N ...
Calcolare il pH e la concentrazione di OH- in una soluzione 0.001 N ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Calco<strong>la</strong>re</strong> <strong>il</strong> <strong>pH</strong> <strong>di</strong> <strong>una</strong> <strong>soluzione</strong> ottenuta mesco<strong>la</strong>ndo20 mL <strong>di</strong> CH 3 CO<strong>OH</strong> 0.5 M e 10 mL <strong>di</strong> CH 3 COONa 0.7 MV Tot = 30 mL[CH 3 CO<strong>OH</strong>][CH 3 COONa]= 0.5 x 20 / 30 = 0.333 M= 0.7 x 10 / 30 = 0.233 M<strong>pH</strong> (tampone) = pK A – log 0.333/0.233 = 4.57Si aggiungono 200 ml <strong>di</strong> acqua. Ricalco<strong>la</strong>re <strong>il</strong> <strong>pH</strong>.VTot = 230 mL 4.73non <strong>di</strong>pendePH(tampone) = pKA – log nacido/nsale = 4.57 dal volume !Si aggiungono 10 ml <strong>di</strong> Na<strong>OH</strong> 0.1 N. Ricalco<strong>la</strong>re <strong>il</strong> <strong>pH</strong>.CH3CO<strong>OH</strong> + <strong>OH</strong> - à CH3COO - + H2O<strong>in</strong>(mmoli) 10 1 7 --reag. -1 -1 +1 +1f<strong>in</strong>ali +9 ≈ 0 +8 +1Tampone acido: <strong>pH</strong> = 4.73 – log(9/8) = 4.68Si aggiungono 20 ml <strong>di</strong> HCl 0.1 N. Ricalco<strong>la</strong>re <strong>il</strong> <strong>pH</strong>.CH3COO - + H + à CH3CO<strong>OH</strong><strong>in</strong>(mmoli) +8 2 9reag. -2 -2 +2f<strong>in</strong>ali +6 ≈ 0 +11Tampone acido: <strong>pH</strong> = 4.73 – log(11/6) = 4.47