Naming and Formula Writing Test
Naming and Formula Writing Test
Naming and Formula Writing Test
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Naming</strong> <strong>and</strong> <strong>Formula</strong> <strong>Writing</strong> <strong>Test</strong><br />
Write the correct formula:<br />
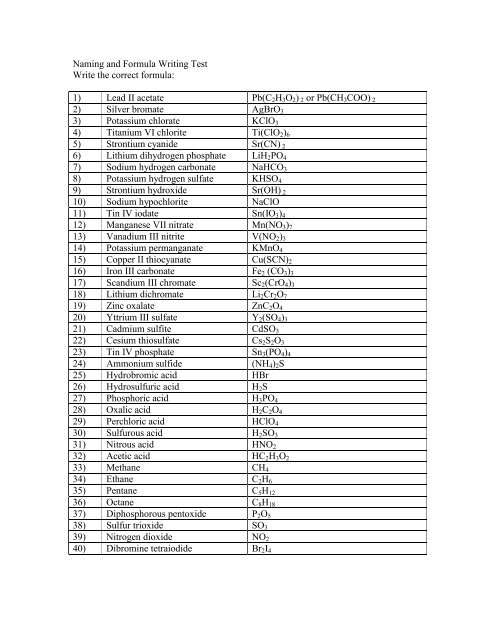
1) Lead II acetate Pb(C 2 H 3 O 2 ) 2 or Pb(CH 3 COO) 2<br />
2) Silver bromate AgBrO 3<br />
3) Potassium chlorate KClO 3<br />
4) Titanium VI chlorite Ti(ClO 2 ) 6<br />
5) Strontium cyanide Sr(CN) 2<br />
6) Lithium dihydrogen phosphate LiH 2 PO 4<br />
7) Sodium hydrogen carbonate NaHCO 3<br />
8) Potassium hydrogen sulfate KHSO 4<br />
9) Strontium hydroxide Sr(OH) 2<br />
10) Sodium hypochlorite NaClO<br />
11) Tin IV iodate Sn(IO 3 ) 4<br />
12) Manganese VII nitrate Mn(NO 3 ) 7<br />
13) Vanadium III nitrite V(NO 2 ) 3<br />
14) Potassium permanganate KMnO 4<br />
15) Copper II thiocyanate Cu(SCN) 2<br />
16) Iron III carbonate Fe 2 (CO 3 ) 3<br />
17) Sc<strong>and</strong>ium III chromate Sc 2 (CrO 4 ) 3<br />
18) Lithium dichromate Li 2 Cr 2 O 7<br />
19) Zinc oxalate ZnC 2 O 4<br />
20) Yttrium III sulfate Y 2 (SO 4 ) 3<br />
21) Cadmium sulfite CdSO 3<br />
22) Cesium thiosulfate Cs 2 S 2 O 3<br />
23) Tin IV phosphate Sn 3 (PO 4 ) 4<br />
24) Ammonium sulfide (NH 4 ) 2 S<br />
25) Hydrobromic acid HBr<br />
26) Hydrosulfuric acid H 2 S<br />
27) Phosphoric acid H 3 PO 4<br />
28) Oxalic acid H 2 C 2 O 4<br />
29) Perchloric acid HClO 4<br />
30) Sulfurous acid H 2 SO 3<br />
31) Nitrous acid HNO 2<br />
32) Acetic acid HC 2 H 3 O 2<br />
33) Methane CH 4<br />
34) Ethane C 2 H 6<br />
35) Pentane C 5 H 12<br />
36) Octane C 8 H 18<br />
37) Diphosphorous pentoxide P 2 O 5<br />
38) Sulfur trioxide SO 3<br />
39) Nitrogen dioxide NO 2<br />
40) Dibromine tetraiodide Br 2 I 4
Write the correct name (remember roman numerals if appropriate):<br />
41) N 2 O 4 Dinitrogen tetroxide<br />
42) SO 2 Sulfur dioxide<br />
43) P 4 O 10 Tetraphosphorus decoxide<br />
44) CO 2 Carbon dioxide<br />
45) Cl 4 Br 8 Tetrachlorine octabromide<br />
46) H 2 O 2 Hydrogen peroxide or dihydrogen dioxide<br />
47) C 4 H 10 Butane<br />
48) C 8 H 18 Octane<br />
49) C 10 H 22 Decane<br />
50) H 2 SO 4 Sulfuric acid<br />
51) H 2 SO 3 Sulfurous acid<br />
52) HNO 3 Nitric acid<br />
53) H 2 CO 3 Carbonic acid<br />
54) HBr Hydrobromic acid<br />
55) HI Hydroiodic acid<br />
56) HF Hydrofluoric acid<br />
57) H 3 N Hydronitric acid<br />
58) Mg(CH 3 COO) 2 Magnesium acetate<br />
59) KClO Potassium hypochlorite<br />
60) AgCN Silver cyanide<br />
61) Fe(NO 3 ) 3 Iron III nitrate<br />
62) KSCN Potassium thiocyanate<br />
63) RbIO 3 Rubidium iodate<br />
64) W 2 (SO 4 ) 5 Tungsten V sulfate<br />
65) NiCO 3 Nickel II carbonate<br />
66) V 2 (Cr 2 O 7 ) 5 Vanadium V dichromate<br />
67) Li 2 C 2 O 4 Lithium oxalate<br />
68) Co 2 (S 2 O 3 ) 3 Cobalt III thiosulfate<br />
69) TiSO 3 Titanium II sulfite<br />
70) Hg 2 O 2 Mercury I oxide<br />
71) HgO Mercury II oxide<br />
72) BaHPO 4 Barium hydrogen phosphate<br />
73) Ba(OH) 2 Barium hydroxide<br />
74) W 3 (PO 4 ) 7 Tungsten VII phosphate<br />
75) Pb(NO 3 ) 4 Lead IV nitrate<br />
76) SnCO 3 Tin II carbonate<br />
77) NH 4 Cl Ammonium chloride<br />
78) ZnCl 2 Zinc chloride<br />
79) (NH 4 ) 2 CO 3 Ammonium carbonate
<strong>Naming</strong> <strong>and</strong> <strong>Formula</strong> <strong>Writing</strong> <strong>Test</strong><br />
Write the correct formula:<br />
1. Lead II acetate<br />
2. Silver bromate<br />
3. Potassium chlorate<br />
4. Titanium VI chlorite<br />
5. Strontium cyanide<br />
6. Lithium dihydrogen phosphate<br />
7. Sodium hydrogen carbonate<br />
8. Potassium hydrogen sulfate<br />
9. Strontium hydroxide<br />
10. Sodium hypochlorite<br />
11. Tin IV iodate<br />
12. Manganese VII nitrate<br />
13. Vanadium III nitrite<br />
14. Potassium permanganate<br />
15. Copper II thiocyanate<br />
16. Iron III carbonate<br />
17. Sc<strong>and</strong>ium III chromate<br />
18. Lithium dichromate<br />
19. Zinc oxalate<br />
20. Yttrium III sulfate<br />
21. Cadmium sulfite<br />
22. Cesium thiosulfate<br />
23. Tin IV phosphate<br />
24. Ammonium sulfide<br />
25. Hydrobromic acid<br />
26. Hydrosulfuric acid<br />
27. Phosphoric acid<br />
28. Oxalic acid<br />
29. Perchloric acid<br />
30. Sulfurous acid<br />
31. Nitrous acid<br />
32. Acetic acid<br />
33. Methane<br />
34. Ethane<br />
35. Pentane<br />
36. Octane<br />
37. Diphosphorous pentoxide<br />
38. Sulfur trioxide<br />
39. Nitrogen dioxide<br />
40. Dibromine tetraiodide
Write the correct name (remember roman numerals if appropriate):<br />
41. N 2 O 4<br />
42. SO 2<br />
43. P 4 O 10<br />
44. CO 2<br />
45. Cl 4 Br 8<br />
46. H 2 O 2<br />
47. C 4 H 10<br />
48. C 8 H 18<br />
49. C 10 H 22<br />
50. H 2 SO 4<br />
51. H 2 SO 3<br />
52. HNO 3<br />
53. H 2 CO 3<br />
54. HBr<br />
55. HI<br />
56. HF<br />
57. H 3 N<br />
58. Mg(CH 3 COO) 2<br />
59. KClO<br />
60. AgCN<br />
61. Fe(NO 3 ) 3<br />
62. KSCN<br />
63. RbIO 3<br />
64. W 2 (SO 4 ) 5<br />
65. NiCO 3<br />
66. V 2 (Cr 2 O 7 ) 5<br />
67. Li 2 C 2 O 4<br />
68. Co 2 (S 2 O 3 ) 3<br />
69. TiSO 3<br />
70. Hg 2 O 2<br />
71. HgO<br />
72. BaHPO 4<br />
73. Ba(OH) 2<br />
74. W 3 (PO 4 ) 7<br />
75. Pb(NO 3 ) 4<br />
76. SnCO 3<br />
77. NH 4 Cl<br />
78. ZnCl 2<br />
79. (NH 4 ) 2 CO 3