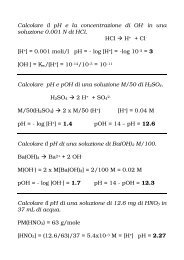
acido
acido
acido
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
V(NaOH), mL [H 3 O + ], M pH<br />
0 0.1 1.00<br />
10 0.0818 1.09<br />
20 0.0667 1.18<br />
30 0.0538 1.27<br />
40 0.0428 1.37<br />
50 0.0333 1.48<br />
60 0.0250 1.60<br />
Acido<br />
70 0.0176 1.75<br />
80 0.0111 1.95<br />
90 0.0053 2.28<br />
95 2.56 × 10 -3 2.59<br />
99 5.02 × 10 -4 3.30<br />
99.9 5.00 × 10 -5 4.30<br />
100 10 -7 7.00<br />
Neutro<br />
100.1 5.00 × 10 -5 * 9.70<br />
101 5.00 × 10 -4 * 10.70<br />
* [OH - ] 110 4.76 × 10 -3 * 11.78 Basico<br />
In eccesso di OH - :<br />
[OH - ] = n(OH - )/V TOT = [C(OH - ) V(NaOH) - 0.1 × 100]/[100 + V(NaOH)]