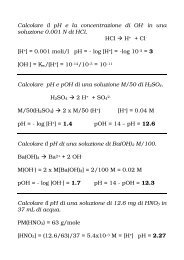
acido
acido
acido
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
3) V(NaOH) = 20 mL<br />
n(H 3 O + ) = n 0 (H 3 O + ) – n(OH - ) = C 0 (H 3 O + )V(HCl) - C 0 (OH - )V(NaOH) =<br />
= 0.1 × 100 mmol – 0.1 × 20 mmol = 8 mmol<br />
V TOT = V 0 + V(NaOH) = 120 mL<br />
[H 3 O + ] = n(H 3 O + )/V TOT = 8/120 = 0.0667 M pH = 1.18<br />
4) V(NaOH) < 100 mL<br />
n(H 3 O + ) = n 0 (H 3 O + ) – n(OH - ) = C 0 (H 3 O + )V(HCl) - C 0 (OH - )V(NaOH) =<br />
= 0.1× 100 mmol – 0.1 × V(NaOH) mmol<br />
V TOT = 100 + V(NaOH) mL<br />
[H 3 O + ] = n(H 3 O + )/V TOT = [10 - 0.1 × V(NaOH)]/[100 + V(NaOH)]