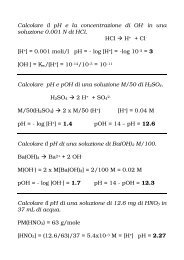
acido
acido
acido
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Titolazione di Acido Forte con Base Forte<br />
Si supponga di dover stimare la variazione di pH in 100 mL di HCl 0.1<br />
M per aggiunta graduale di NaOH 0.1 M. In soluzione, hanno luogo:<br />
HCl (aq)<br />
+ H 2<br />
O (l)<br />
H 3<br />
O + (aq) + Cl- (aq)<br />
NaOH (aq)<br />
Na + (aq) + OH- (aq)<br />
OH - (aq) + H 3 O+ (aq)<br />
2 H 2<br />
O (l)<br />
K = (K -1 14 -2<br />
W ) = 10 M<br />
1) V(NaOH) = 0 mL<br />
[H 3 O + ] = 0.1 M pH = 1<br />
2) V(NaOH) = 10 mL<br />
n(H 3 O + ) = n 0 (H 3 O + ) – n(OH - ) = C 0 (H 3 O + )V(HCl) - C 0 (OH - )V(NaOH) =<br />
= 0.1×100 mmol – 0.1×10 mmol = 9 mmol<br />
V TOT = V 0 + V(NaOH) = 110 mL<br />
[H 3 O + ] = n(H 3 O + )/V TOT = 9/110 = 0.0818 M pH = 1.09