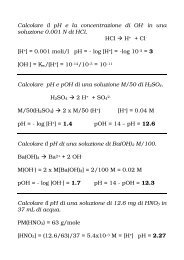
acido
acido
acido
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Si considerino i due equilibri presenti simultaneamente in soluzione acquosa:<br />
HA (aq)<br />
+ H 2<br />
O (l) H 3<br />
O + (aq) + A- (aq)<br />
A - (aq) + H 2 O (l) HA (aq)<br />
+ OH - (aq)<br />
pH i = pK A - log [HA] 0 /[A - ] 0<br />
a) AGGIUNTA di [OH - ]:<br />
HA (aq)<br />
+ OH - (aq) A - (aq) + H 2 O (l)<br />
scompare HA, compare A -<br />
K = [A - ]/[HA][OH - ] = K A /K W >> 1 (10 4 – 10 10 )<br />
pH - - - f = pK A – log ([HA] 0 – [OH ])/([A ] 0 + [OH ])<br />
b) AGGIUNTA di [H 3 O + ]:<br />
A - (aq) + H 3 O+ (aq) HA (aq)<br />
+ H 2<br />
O (l)<br />
scompare A - , compare HA<br />
K = 1/K A >> 1 (10 4 – 10 10 )<br />
pH f = pK A – log ([HA] 0 + [H 3 O + ])/([A - ] 0 - [H 3 O + ])<br />
c) AGGIUNTA di H 2 O:<br />
pH f = pK A – log[n HA /V]/[n A- /V] = pK A – log n HA /n A-