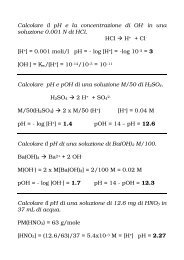
acido
acido
acido
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
III CASO: NH 4 Cl in acqua<br />
H 2<br />
O (l)<br />
NH 4<br />
Cl (s)<br />
NH 4<br />
+<br />
(aq)<br />
+ Cl - (aq)<br />
Comportamento <strong>acido</strong> di NH 4+ : NH<br />
+<br />
4 (aq)<br />
+ H 2<br />
O (l) NH 3(aq)<br />
+ H 3<br />
O + (aq)<br />
K 4+ + 4+ - - A (NH ) = [H 3 O ][NH 3 ]/[NH ] × [OH ]/[OH ] =<br />
= K W [NH 3 ]/[NH 4+ ][OH - ] = K W /K B (NH 3 ) =10 -14 /10 -5 = 10 -9 M<br />
NH 4+ è un <strong>acido</strong> debole di K A = 10 -9 M K B (NH 3 ) × K A (NH 4+ ) = K W = 10 -14 M 2<br />
NH 4 Cl in acqua dà IDROLISI ACIDA e soluzione acida.