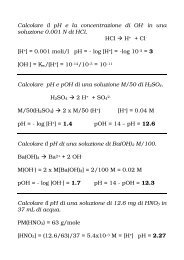
acido
acido
acido
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
pH di Soluzioni Acquose di Basi Deboli Monoprotiche<br />
B (aq)<br />
+ H 2<br />
O (l) OH - (aq) + BH+ (aq) K B = [BH + ][OH - ]/[B]<br />
H 2<br />
O (l)<br />
+ H 2<br />
O (l) OH - (aq) + H 3 O+ (aq)<br />
K W = [H 3 O + ][OH - ] = 10 -14 M 2<br />
[OH - ] TOT<br />
= [OH - ] B<br />
+ [OH - ] Acqua<br />
1 a approssimazione: [OH - ] B >> [OH - ] Acqua [B] 0 ≥ 10 -6 M<br />
2 a approssimazione: [B] eq ≈ [B] 0 K B ≤ 10 -4 M<br />
K B =[BH + ][OH - ] TOT /[B] ≈ [BH + ][OH - ] B /[B] 0 = [OH - ] 2 /[B] 0<br />
[OH - ] = ([B] 0 K B ) 1/2<br />
pOH = -log([B] 0 K B ) 1/2 = -1/2 log([B] 0 ) + 1/2 pK B<br />
pH = 14.00 + 1/2 log([B] 0 ) - 1/2 pK B<br />
2b<br />
Basi deboli concentrate con K B non molto alta (< 10 -4 )