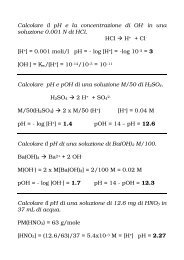
acido
acido
acido
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
pH di Soluzioni Acquose di Basi Forti<br />
B (aq)<br />
+ H 2<br />
O (l) OH - (aq) + BH+ (aq) Completa K B → ∞<br />
H 2<br />
O (l)<br />
+ H 2<br />
O (l)<br />
OH - (aq) + H 3 O+ (aq)<br />
K W = [H 3 O + ][OH - ] = 10 -14 M 2<br />
[OH - ] Acqua ≈ 10 -7 M<br />
Se [OH - ] B >> [OH - ] Acqua ≈ 10 -7 M<br />
[OH - ] TOT ≈ [OH - ] B = z [B] 0<br />
[B] 0 = concentrazione iniziale di base<br />
z = numero di moli di OH - dissociate (per mole di reagente)<br />
pOH = -log (z [B] 0 )<br />
1b<br />
pH = 14 - pOH<br />
Basi forti non diluitissime