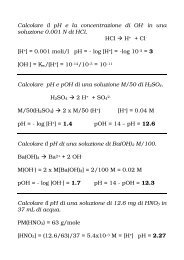
acido
acido
acido
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Scala di pH<br />
pH = – log [H 3 O + ]<br />
Basso pH ≡ alta concentrazione [H 3 O + ] Alto pH ≡ bassa concentrazione [H 3 O + ]<br />
-1 0 7 14 15<br />
pOH = – log [OH - ]<br />
Alto pOH ≡ bassa concentrazione [OH-] Basso pOH ≡ alta concentrazione [OH - ]<br />
15<br />
14<br />
7<br />
0<br />
-1<br />
A 25 °C in soluzione acquosa:<br />
K W = [H 3 O + ][OH - ] = 10 -14 M 2 log K W = log [H 3 O + ] + log[OH - ] pH + pOH = 14<br />
pOH<br />
pH < 7 pH > 7<br />
7<br />
0<br />
pOH > 7<br />
pOH < 7 14<br />
7<br />
14 ACIDO NEUTRO BASICO<br />
0<br />
pH