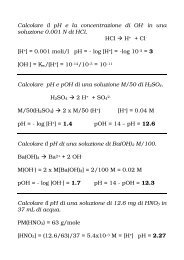
acido
acido
acido
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Costanti di Ionizzazione Acida<br />
HA (aq)<br />
+ H 2<br />
O (l)<br />
H 3<br />
O + (aq) + A- (aq)<br />
K C = [A - ][H 3 O + ]/[HA][H 2 O]<br />
se [H 2 O] ca. costante:<br />
K A = K C [H 2 O] = [A - ][H 3 O + ]/[HA] COSTANTE DI IONIZZAZIONE ACIDA<br />
E.g. 1:<br />
CH 3<br />
COOH (aq)<br />
+ H 2<br />
O (l)<br />
H 3<br />
O + (aq) + CH 3 COO- (aq)<br />
K A = [CH 3 COO - ][H 3 O + ]/[CH 3 COOH] = 1.8×10 -5 M<br />
pK A = - log(K A ) = - log (1.8×10 -5 ) = 4.74<br />
E.g. 2:<br />
HCN (aq)<br />
+ H 2<br />
O (l)<br />
H 3<br />
O + (aq) + CN- (aq)<br />
K A = [CN - ][H 3 O + ]/[HCN] = 4.9×10 -10 M pK A = 9.31