Chemistry 1 HH#7 Naming/Formulas Name each of the following ...
Chemistry 1 HH#7 Naming/Formulas Name each of the following ...
Chemistry 1 HH#7 Naming/Formulas Name each of the following ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Chemistry</strong> 1 <strong>HH#7</strong> <strong>Naming</strong>/<strong>Formulas</strong><br />
<strong>Name</strong> <strong>each</strong> <strong>of</strong> <strong>the</strong> <strong>following</strong> compounds.<br />
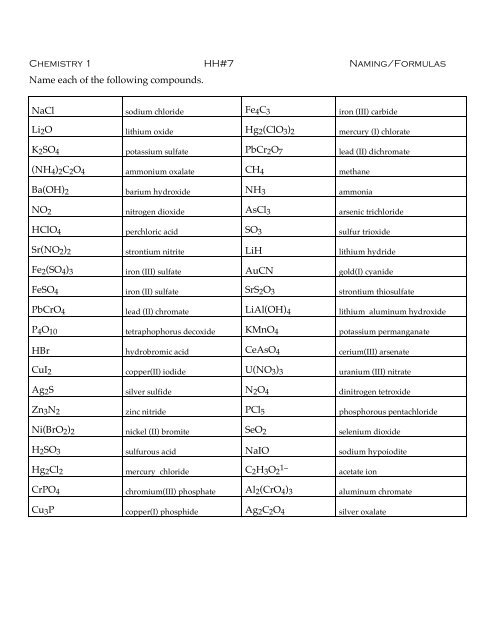
NaCl sodium chloride Fe4C3 iron (III) carbide<br />
Li2O lithium oxide Hg2(ClO3)2 mercury (I) chlorate<br />
K2SO4 potassium sulfate PbCr2O7 lead (II) dichromate<br />
(NH4)2C2O4 ammonium oxalate CH4 methane<br />
Ba(OH)2 barium hydroxide NH3 ammonia<br />
NO2 nitrogen dioxide AsCl3 arsenic trichloride<br />
HClO4 perchloric acid SO3 sulfur trioxide<br />
Sr(NO2)2 strontium nitrite LiH lithium hydride<br />
Fe2(SO4)3 iron (III) sulfate AuCN gold(I) cyanide<br />
FeSO4 iron (II) sulfate SrS2O3 strontium thiosulfate<br />
PbCrO4 lead (II) chromate LiAl(OH)4 lithium aluminum hydroxide<br />
P4O10 tetraphophorus decoxide KMnO4 potassium permanganate<br />
HBr hydrobromic acid CeAsO4 cerium(III) arsenate<br />
CuI2 copper(II) iodide U(NO3)3 uranium (III) nitrate<br />
Ag2S silver sulfide N2O4 dinitrogen tetroxide<br />
Zn3N2 zinc nitride PCl5 phosphorous pentachloride<br />
Ni(BrO2)2 nickel (II) bromite SeO2 selenium dioxide<br />
H2SO3 sulfurous acid NaIO sodium hypoiodite<br />
Hg2Cl2 mercury chloride C2H3O2 1– acetate ion<br />
CrPO4 chromium(III) phosphate Al2(CrO4)3 aluminum chromate<br />
Cu3P copper(I) phosphide Ag2C2O4 silver oxalate
Write <strong>the</strong> correct chemical formula for <strong>each</strong> <strong>of</strong> <strong>the</strong> <strong>following</strong> compounds.<br />
lithium nitride Li3N iron(III) phosphite FePO3<br />
tin(IV) fluoride SnF4 hydriodic acid HI<br />
sulfurous acid H2SO3 lead(II) nitrite Pb(NO2)2<br />
dinitrogen tetrasulfide N2S4 potassium carbonate K2CO3<br />
sodium hydroxide NaOH chromium(VI) oxide CrO3<br />
acetic acid HC2H3O2 potassium permanganate KMnO4<br />
ammonium thiosulfate (NH4)2S2O3 bismuth(III) arsenate BiAsO4<br />
ice H2O sulfur hexafluoride SF6<br />
mercury(I) nitrate Hg2(NO3)2 mercury(II) carbonate HgCO3<br />
zinc chlorite Zn(ClO2)2 hydrochloric acid HCl<br />
calcium hypoiodite Ca(IO)2 copper(I) selenide Cu2Se<br />
copper(I) iodide CuI perchloric acid HClO4<br />
lead(IV) phosphate Pb3(PO4)4 tin(IV) chloride SnCl4<br />
aluminum hydroxide Al(OH)3 diphosphorus oxide P2O<br />
silicon dioxide SiO2 zinc bicarbonate Zn(HCO3)2<br />
chromium(III) carbide Cr4C3 magnesium nitrate Mg(NO3)2<br />
ammonium phosphite (NH4)3PO3 methane CH4<br />
copper(I) nitrite CuNO2 cadmium(II) arsenite Cd3(AsO3)2<br />
scandium(III) silicide Sc4Si3 snow H2O<br />
iron(III) cyanide Fe(CN)3 germanium diiodide GeI2<br />
barium thiosulfate BaS2O3 vanadium(V) sulfide V2S5