Polyatomic Ions - Chandler-Gilbert Community College
Polyatomic Ions - Chandler-Gilbert Community College
Polyatomic Ions - Chandler-Gilbert Community College
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
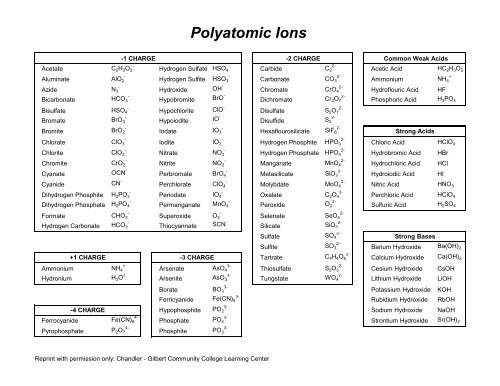
-<br />
Acetate C2H3O2 -<br />
Aluminate AlO2 -<br />
Azide N3 Bicarbonate<br />
-<br />
HCO3 -<br />
Bisulfate HSO4 Bromate<br />
Bromite<br />
-<br />
BrO3 -<br />
BrO2 -<br />
Chlorate ClO3 -<br />
Chlorite ClO2 -<br />
Chromite CrO2 Cyanate<br />
Cyanide<br />
OCN -<br />
CN -<br />
-<br />
Dihydrogen Phosphite H2PO3 Dihydrogen Phosphate H 2PO 4<br />
-<br />
Formate CHO2 Hydrogen Carbonate<br />
-<br />
HCO3 Ammonium<br />
+<br />
NH4 Hydronium<br />
H3O +<br />
Ferrocyanide<br />
Pyrophosphate<br />
+1 CHARGE<br />
-4 CHARGE<br />
4-<br />
Fe(CN) 6<br />
4-<br />
P2O7 -1 CHARGE<br />
-<br />
<strong>Polyatomic</strong> <strong>Ions</strong><br />
-<br />
Hydrogen Sulfate HSO4 -<br />
Hydrogen Sulfite HSO3 Hydroxide<br />
Hypobromite<br />
Hypochlorite<br />
Hypoiodite<br />
Iodate<br />
OH -<br />
BrO -<br />
ClO -<br />
IO -<br />
-<br />
IO3 -<br />
Iodite IO2 -<br />
Nitrate NO3 -<br />
Nitrite NO2 -<br />
Perbromate BrO4 -<br />
Perchlorate ClO4 -<br />
Periodate IO4 Permanganate<br />
-<br />
MnO4 -<br />
Superoxide O2 Thiocyannate<br />
SCN -<br />
3-<br />
Arsenate AsO4 Arsenite<br />
3-<br />
AsO3 3-<br />
Borate BO3 Ferricyanide<br />
Hypophosphite<br />
Phosphate<br />
Phosphite<br />
-3 CHARGE<br />
3-<br />
Fe(CN) 6<br />
3-<br />
PO2 3-<br />
PO4 3-<br />
PO3 Reprint with permission only: <strong>Chandler</strong> - <strong>Gilbert</strong> <strong>Community</strong> <strong>College</strong> Learning Center<br />
2-<br />
Carbide C2 2-<br />
Carbonate CO3 Chromate<br />
2-<br />
CrO4 Dichromate<br />
2-<br />
Cr2O7 2-<br />
Disulfate S2O7 Disulfide<br />
Hexaflourosilicate<br />
2-<br />
S2 2-<br />
SiF6 2-<br />
Hydrogen Phosphite HPO3 2-<br />
Hydrogen Phosphate HPO4 2-<br />
Manganate MnO4 2-<br />
Metasilicate SiO3 2-<br />
Molybdate MoO4 2-<br />
Oxalate C2O4 Peroxide<br />
2-<br />
O2 2-<br />
Selenate SeO4 Silicate<br />
Sulfate<br />
Sulfite<br />
Tartrate<br />
2-<br />
SiO3 2-<br />
SO4 2-<br />
SO3 2-<br />
C4H4O6 2-<br />
Thiosulfate S2O3 Tungstate<br />
-2 CHARGE<br />
2-<br />
WO4 Common Weak Acids<br />
Acetic Acid HC 2H 3O 2<br />
+<br />
Ammonium NH4 Hydroflouric Acid HF<br />
Phosphoric Acid H3PO4 Strong Acids<br />
Chloric Acid HClO 3<br />
Hydrobromic Acid HBr<br />
Hydrochloric Acid HCl<br />
Hydroiodic Acid HI<br />
Nitric Acid HNO 3<br />
Perchloric Acid HClO4 Sulfuric Acid H2SO4 Strong Bases<br />
Barium Hydroxide<br />
Calcium Hydroxide<br />
Ba(OH) 2<br />
Ca(OH) 2<br />
Cesium Hydroxide CsOH<br />
Lithium Hydroxide LiOH<br />
Potassium Hydroxide KOH<br />
Rubidium Hydroxide RbOH<br />
Sodium Hydroxide NaOH<br />
Strontium Hydroxide Sr(OH) 2
Acetate<br />
-<br />
C2H3O2 Aluminate<br />
-<br />
AlO2 Ammonium<br />
+<br />
NH4 Arsenate<br />
3-<br />
AsO4 Arsenite<br />
3-<br />
AsO3 Azide<br />
-<br />
N3 Bicarbonate<br />
-<br />
HCO3 Bisulfate<br />
-<br />
HSO4 Borate<br />
3-<br />
BO3 Bromate<br />
-<br />
BrO3 Bromite<br />
-<br />
BrO2 Carbide<br />
2-<br />
C2 Carbonate<br />
2-<br />
CO3 Chlorate<br />
-<br />
ClO3 Chlorite<br />
-<br />
ClO2 Chromate<br />
2-<br />
CrO4 Chromite<br />
-<br />
CrO2 Cyanate<br />
OCN -<br />
CN -<br />
Cyanide<br />
Dichromate<br />
2-<br />
Cr2O7 Dihydrogen Phosphate<br />
-<br />
H2PO4 Dihydrogen Phosphite<br />
-<br />
H2PO3 <strong>Polyatomic</strong> <strong>Ions</strong><br />
Disulfate<br />
2-<br />
S2O7 Disulfide<br />
2-<br />
S2 Ferricyanide<br />
3-<br />
Fe(CN) 6<br />
Ferrocyanide<br />
4-<br />
Fe(CN) 6<br />
Formate<br />
-<br />
CHO2 Hexaflourosilicate<br />
2-<br />
SiF6 Hydrogen Carbonate<br />
-<br />
HCO3 Hydrogen Phosphate<br />
2-<br />
HPO4 Hydrogen Phosphite<br />
2-<br />
HPO3 Hydrogen Sulfate<br />
-<br />
HSO4 Hydrogen Sulfite<br />
-<br />
HSO3 Hydronium H3O +<br />
Hydroxide<br />
Hypobromite<br />
Hypochlorite<br />
OH -<br />
BrO -<br />
ClO -<br />
IO -<br />
Hypoiodite<br />
Hypophosphite<br />
3-<br />
PO2 Iodate<br />
-<br />
IO3 Iodite<br />
-<br />
IO2 Manganate<br />
2-<br />
MnO4 Metasilicate<br />
2-<br />
SiO3 Reprint with permission only: <strong>Chandler</strong> - <strong>Gilbert</strong> <strong>Community</strong> <strong>College</strong> Learning Center<br />
Molybdate<br />
2-<br />
MoO4 Nitrate<br />
-<br />
NO3 Nitrite<br />
-<br />
NO2 Oxalate<br />
2-<br />
C2O4 Perbromate<br />
-<br />
BrO4 Perchlorate<br />
-<br />
ClO4 Periodate<br />
-<br />
IO4 Permanganate<br />
-<br />
MnO4 Peroxide<br />
2-<br />
O2 Phosphate<br />
3-<br />
PO4 Phosphite<br />
3-<br />
PO3 Pyrophosphate<br />
4-<br />
P2O7 Selenate<br />
2-<br />
SeO4 Silicate<br />
2-<br />
SiO3 Sulfate<br />
2-<br />
SO4 Sulfite<br />
2-<br />
SO3 Superoxide<br />
-<br />
O2 Tartrate<br />
2-<br />
C4H4O6 SCN -<br />
Thiocyannate<br />
Thiosulfate<br />
2-<br />
S2O3 Tungstate<br />
2-<br />
WO4