Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
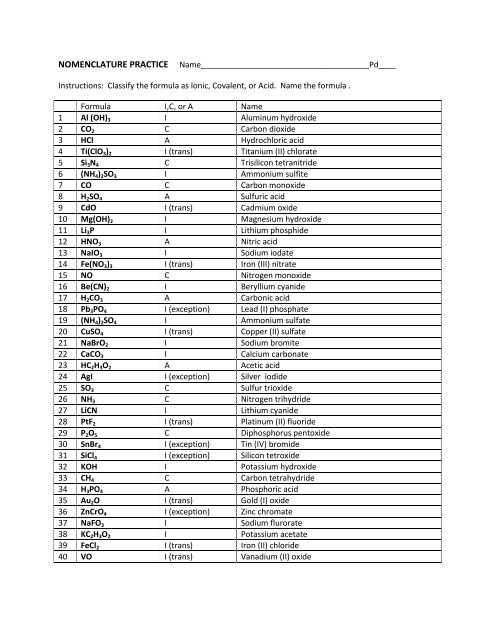
<strong>NOMENCLATURE</strong> <strong>PRACTICE</strong> Name______________________________________Pd____<br />
Instructions: Classify the formula as Ionic, Covalent, or Acid. Name the formula .<br />
Formula I,C, or A Name<br />
1 Al (OH)3 I Aluminum hydroxide<br />
2 CO2 C Carbon dioxide<br />
3 HCl A Hydrochloric acid<br />
4 Ti(ClO3)2 I (trans) Titanium (II) chlorate<br />
5 Si3N4 C Trisilicon tetranitride<br />
6 (NH4)2SO3 I Ammonium sulfite<br />
7 CO C Carbon monoxide<br />
8 H2SO4 A Sulfuric acid<br />
9 CdO I (trans) Cadmium oxide<br />
10 Mg(OH)2 I Magnesium hydroxide<br />
11 Li3P I Lithium phosphide<br />
12 HNO3 A Nitric acid<br />
13 NaIO3 I Sodium iodate<br />
14 Fe(NO3)3 I (trans) Iron (III) nitrate<br />
15 NO C Nitrogen monoxide<br />
16 Be(CN)2 I Beryllium cyanide<br />
17 H2CO3 A Carbonic acid<br />
18 Pb3PO4 I (exception) Lead (I) phosphate<br />
19 (NH4)2SO4 I Ammonium sulfate<br />
20 CuSO4 I (trans) Copper (II) sulfate<br />
21 NaBrO2 I Sodium bromite<br />
22 CaCO3 I Calcium carbonate<br />
23 HC2H3O2 A Acetic acid<br />
24 AgI I (exception) Silver iodide<br />
25 SO3 C Sulfur trioxide<br />
26 NH3 C Nitrogen trihydride<br />
27 LiCN I Lithium cyanide<br />
28 PtF2 I (trans) Platinum (II) fluoride<br />
29 P2O5 C Diphosphorus pentoxide<br />
30 SnBr4 I (exception) Tin (IV) bromide<br />
31 SiCl4 I (exception) Silicon tetroxide<br />
32 KOH I Potassium hydroxide<br />
33 CH4 C Carbon tetrahydride<br />
34 H3PO4 A Phosphoric acid<br />
35 Au2O I (trans) Gold (I) oxide<br />
36 ZnCrO4 I (exception) Zinc chromate<br />
37 NaFO3 I Sodium flurorate<br />
38 KC2H3O2 I Potassium acetate<br />
39 FeCl2 I (trans) Iron (II) chloride<br />
40 VO I (trans) Vanadium (II) oxide
Write the Formula for the following substances:<br />
1 Sodium chloride NaCl<br />
2 Tin (II) oxide SnO<br />
3 Diphosphorus trioxide P2O5<br />
4 Hydrochloric acid HCl<br />
5 Nitrogen Dioxide NO2<br />
6 Iron (III) sulfate Fe(SO4)3<br />
7 Barium Nitrate Ba(NO3)2<br />
8 Potassium Nitrite KNO3<br />
9 Sulfuric Acid H2SO4<br />
10 Silver Chromate Ag2CrO4<br />
11 Lithium Acetate LiC2H3O2<br />
12 Strontium Fluoride SrF2<br />
13 Calcium Fluorate Ca(FO3)2<br />
14 Barium Fluorite Ba(FO2)2<br />
15 Phosphoric Acid H3PO4<br />
16 Calcium Hydroxide Ca(OH)2<br />
17 Vanadium (I) Sulfate VSO4<br />
18 Ammonium Iodide NH4I<br />
19 Dicarbon Hexahydride C2H6<br />
20 Lithium Phosphide Li3P<br />
21 Carbonic Acid H2CO3<br />
22 Zinc Chloride ZnCl2<br />
23 Ammonium Nitrate NH4NO3<br />
24 Carbon Tetrachloride CCl4<br />
25 Aluminum Iodate Al(IO3)3<br />
26 Sodium Bromate NaBr<br />
27 Barium Bromide BaBr2<br />
28 Beryllium Iodide BeI2<br />
29 Nitric Acid HNO3<br />
30 Ammonium Phosphate (NH4)3PO4<br />
31 Cadmium Nitrate Cd(NO3)2<br />
32 Nitrogen Trifluoride NF3<br />
33 Gallium Oxide GaO<br />
34 Sulfur Dioxide SO2<br />
35 Sulfurous Acid H2SO3<br />
36 Sodium Phosphate Na3PO4<br />
37 Silver Oxide Ag2O<br />
38 Carbon Dioxide CO2<br />
39 Aluminum Nitrate Al(NO3)3<br />
40 Acetic Acid HC2H3O2