maenas (intertidal zone) and Segonzacia mesatlantica - Station ...
maenas (intertidal zone) and Segonzacia mesatlantica - Station ...
maenas (intertidal zone) and Segonzacia mesatlantica - Station ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
166 Current Protein <strong>and</strong> Peptide Science, 2008, Vol. 9, No. 2 Bruneaux et al.<br />
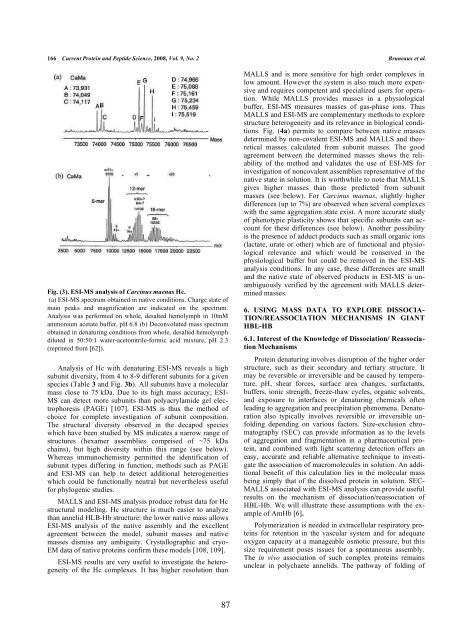
Fig. (3). ESI-MS analysis of Carcinus <strong>maenas</strong> Hc.<br />
(a) ESI-MS spectrum obtained in native conditions. Charge state of<br />
main peaks <strong>and</strong> magnification are indicated on the spectrum.<br />
Analysis was performed on whole, desalted hemolymph in 10mM<br />
ammonium acetate buffer, pH 6.8 (b) Deconvoluted mass spectrum<br />
obtained in denaturing conditions from whole, desalted hemolymph<br />
diluted in 50:50:1 water-acetonitrile-formic acid mixture, pH 2.3<br />
(reprinted from [62]).<br />
Analysis of Hc with denaturing ESI-MS reveals a high<br />
subunit diversity, from 4 to 8-9 different subunits for a given<br />
species (Table 3 <strong>and</strong> Fig. 3b). All subunits have a molecular<br />
mass close to 75 kDa. Due to its high mass accuracy, ESI-<br />
MS can detect more subunits than polyacrylamide gel electrophoresis<br />
(PAGE) [107]. ESI-MS is thus the method of<br />
choice for complete investigation of subunit composition.<br />
The structural diversity observed in the decapod species<br />
which have been studied by MS indicates a narrow range of<br />
structures (hexamer assemblies comprised of ~75 kDa<br />
chains), but high diversity within this range (see below).<br />
Whereas immunochemistry permitted the identification of<br />
subunit types differing in function, methods such as PAGE<br />
<strong>and</strong> ESI-MS can help to detect additional heterogeneities<br />
which could be functionally neutral but nevertheless useful<br />
for phylogenic studies.<br />
MALLS <strong>and</strong> ESI-MS analysis produce robust data for Hc<br />
structural modeling. Hc structure is much easier to analyze<br />
than annelid HLB-Hb structure: the lower native mass allows<br />
ESI-MS analysis of the native assembly <strong>and</strong> the excellent<br />
agreement between the model, subunit masses <strong>and</strong> native<br />
masses dismiss any ambiguity. Crystallographic <strong>and</strong> cryo-<br />
EM data of native proteins confirm these models [108, 109].<br />
ESI-MS results are very useful to investigate the heterogeneity<br />
of the Hc complexes. It has higher resolution than<br />
MALLS <strong>and</strong> is more sensitive for high order complexes in<br />
low amount. However the system is also much more expensive<br />
<strong>and</strong> requires competent <strong>and</strong> specialized users for operation.<br />
While MALLS provides masses in a physiological<br />
buffer, ESI-MS measures masses of gas-phase ions. Thus<br />
MALLS <strong>and</strong> ESI-MS are complementary methods to explore<br />
structure heterogeneity <strong>and</strong> its relevance in biological conditions.<br />
Fig. (4a) permits to compare between native masses<br />
determined by non-covalent ESI-MS <strong>and</strong> MALLS <strong>and</strong> theoretical<br />
masses calculated from subunit masses. The good<br />
agreement between the determined masses shows the reliability<br />
of the method <strong>and</strong> validates the use of ESI-MS for<br />
investigation of noncovalent assemblies representative of the<br />
native state in solution. It is worthwhile to note that MALLS<br />
gives higher masses than those predicted from subunit<br />
masses (see below). For Carcinus <strong>maenas</strong>, slightly higher<br />
differences (up to 7%) are observed when several complexes<br />
with the same aggregation state exist. A more accurate study<br />
of phenotypic plasticity shows that specific subunits can account<br />
for these differences (see below). Another possibility<br />
is the presence of adduct products such as small organic ions<br />
(lactate, urate or other) which are of functional <strong>and</strong> physiological<br />
relevance <strong>and</strong> which would be conserved in the<br />
physiological buffer but could be removed in the ESI-MS<br />
analysis conditions. In any case, these differences are small<br />
<strong>and</strong> the native state of observed products in ESI-MS is unambiguously<br />
verified by the agreement with MALLS determined<br />
masses.<br />
6. USING MASS DATA TO EXPLORE DISSOCIA-<br />
TION/REASSOCIATION MECHANISMS IN GIANT<br />
HBL-HB<br />
6.1. Interest of the Knowledge of Dissociation/ Reassociation<br />
Mechanisms<br />
Protein denaturing involves disruption of the higher order<br />
structure, such as their secondary <strong>and</strong> tertiary structure. It<br />
may be reversible or irreversible <strong>and</strong> be caused by temperature,<br />
pH, shear forces, surface area changes, surfactants,<br />
buffers, ionic strength, freeze-thaw cycles, organic solvents,<br />
<strong>and</strong> exposure to interfaces or denaturing chemicals often<br />
leading to aggregation <strong>and</strong> precipitation phenomena. Denaturation<br />
also typically involves reversible or irreversible unfolding<br />
depending on various factors. Size-exclusion chromatography<br />
(SEC) can provide information as to the levels<br />
of aggregation <strong>and</strong> fragmentation in a pharmaceutical protein,<br />
<strong>and</strong> combined with light scattering detection offers an<br />
easy, accurate <strong>and</strong> reliable alternative technique to investigate<br />
the association of macromolecules in solution. An additional<br />
benefit of this calculation lies in the molecular mass<br />
being simply that of the dissolved protein in solution. SEC-<br />
MALLS associated with ESI-MS analysis can provide useful<br />
results on the mechanism of dissociation/reassociation of<br />
HBL-Hb. We will illustrate these assumptions with the example<br />
of AmHb [6].<br />
Polymerization is needed in extracellular respiratory proteins<br />
for retention in the vascular system <strong>and</strong> for adequate<br />
oxygen capacity at a manageable osmotic pressure, but this<br />
size requirement poses issues for a spontaneous assembly.<br />
The in vivo association of such complex proteins remains<br />
unclear in polychaete annelids. The pathway of folding of<br />
87