SMQ-V047 N-002_ligas_size.pdf - Journal of the Mexican Chemical ...
SMQ-V047 N-002_ligas_size.pdf - Journal of the Mexican Chemical ...
SMQ-V047 N-002_ligas_size.pdf - Journal of the Mexican Chemical ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Preparation <strong>of</strong> 11-Hydroxylated 11,13-Dihydrosesquiterpene Lactones 135<br />
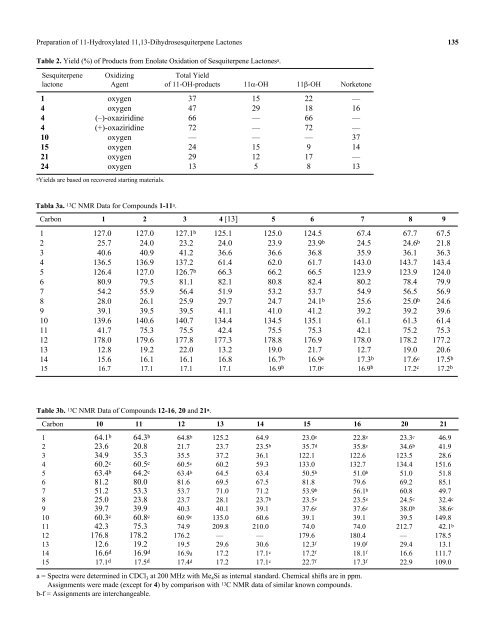
Table 2. Yield (%) <strong>of</strong> Products from Enolate Oxidation <strong>of</strong> Sesquiterpene Lactones a .<br />
Sesquiterpene Oxidizing Total Yield<br />
lactone Agent <strong>of</strong> 11-OH-products 11α-OH 11β-OH Norketone<br />
1 oxygen 37 15 22 —<br />
4 oxygen 47 29 18 16<br />
4 (–)-oxaziridine 66 — 66 —<br />
4 (+)-oxaziridine 72 — 72 —<br />
10 oxygen — — — 37<br />
15 oxygen 24 15 9 14<br />
21 oxygen 29 12 17 —<br />
24 oxygen 13 5 8 13<br />
aYields are based on recovered starting materials.<br />
Tabla 3a. 13 C NMR Data for Compounds 1-11 a .<br />
Carbon 1 2 3 4[13] 5 6 7 8 9<br />
1 127.0 127.0 127.1 b 125.1 125.0 124.5 67.4 67.7 67.5<br />
2 25.7 24.0 23.2 24.0 23.9 23.9 b 24.5 24.6 b 21.8<br />
3 40.6 40.9 41.2 36.6 36.6 36.8 35.9 36.1 36.3<br />
4 136.5 136.9 137.2 61.4 62.0 61.7 143.0 143.7 143.4<br />
5 126.4 127.0 126.7 b 66.3 66.2 66.5 123.9 123.9 124.0<br />
6 80.9 79.5 81.1 82.1 80.8 82.4 80.2 78.4 79.9<br />
7 54.2 55.9 56.4 51.9 53.2 53.7 54.9 56.5 56.9<br />
8 28.0 26.1 25.9 29.7 24.7 24.1 b 25.6 25.0 b 24.6<br />
9 39.1 39.5 39.5 41.1 41.0 41.2 39.2 39.2 39.6<br />
10 139.6 140.6 140.7 134.4 134.5 135.1 61.1 61.3 61.4<br />
11 41.7 75.3 75.5 42.4 75.5 75.3 42.1 75.2 75.3<br />
12 178.0 179.6 177.8 177.3 178.8 176.9 178.0 178.2 177.2<br />
13 12.8 19.2 22.0 13.2 19.0 21.7 12.7 19.0 20.6<br />
14 15.6 16.1 16.1 16.8 16.7 b 16.9 c 17.3 b 17.6 c 17.5 b<br />
15 16.7 17.1 17.1 17.1 16.9 b 17.0 c 16.9 b 17.2 c 17.2 b<br />
Table 3b. 13 C NMR Data <strong>of</strong> Compounds 12-16, 20 and 21 a .<br />
Carbon 10 11 12 13 14 15 16 20 21<br />
1 64.1 b 64.3 b 64.8 b 125.2 64.9 23.0 e 22.8 e 23.3 c 46.9<br />
2 23.6 20.8 21.7 23.7 23.5 b 35.7 d 35.8 c 34.6 b 41.9<br />
3 34.9 35.3 35.5 37.2 36.1 122.1 122.6 123.5 28.6<br />
4 60.2 c 60.5 c 60.5 c 60.2 59.3 133.0 132.7 134.4 151.6<br />
5 63.4 b 64.2 c 63.4 b 64.5 63.4 50.5 b 51.0 b 51.0 51.8<br />
6 81.2 80.0 81.6 69.5 67.5 81.8 79.6 69.2 85.1<br />
7 51.2 53.3 53.7 71.0 71.2 53.9 b 56.1 b 60.8 49.7<br />
8 25.0 23.8 23.7 28.1 23.7 b 23.5 e 23.5 e 24.5 c 32.4 c<br />
9 39.7 39.9 40.3 40.1 39.1 37.6 c 37.6 c 38.0 b 38.6 c<br />
10 60.3 c 60.8 c 60.9 c 135.0 60.6 39.1 39.1 39.5 149.8<br />
11 42.3 75.3 74.9 209.8 210.0 74.0 74.0 212.7 42.1 b<br />
12 176.8 178.2 176.2 — — 179.6 180.4 — 178.5<br />
13 12.6 19.2 19.5 29.6 30.6 12.3 f 19.0 f 29.4 13.1<br />
14 16.6 d 16.9 d 16.9d 17.2 17.1 c 17.2 f 18.1 f 16.6 111.7<br />
15 17.1 d 17.5 d 17.4 d 17.2 17.1 c 22.7 f 17.3 f 22.9 109.0<br />
a = Spectra were determined in CDCl 3 at 200 MHz with Me 4 Si as internal standard. <strong>Chemical</strong> shifts are in ppm.<br />
Assignments were made (except for 4) by comparison with 13 C NMR data <strong>of</strong> similar known compounds.<br />
b-f = Assignments are interchangeable.