world cancer report - iarc
world cancer report - iarc
world cancer report - iarc
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
HUMAN PAPILLOMAVIRUS VACCINATION<br />
SUMMARY<br />
>Infection with human papillomaviruses<br />
is common and causes some benign<br />
lesions as well as cervical and other <strong>cancer</strong>s.<br />
> Vaccines based on human papillomaviruses<br />
may be prophylactic, therapeutic<br />
or a combination of both, and are<br />
potentially a safe and effective means of<br />
preventing or controlling disease.<br />
> Technical difficulties associated with the<br />
development of such vaccines are considerable,<br />
but several phase I, II and III<br />
trials are under way.<br />
From a public health perspective, there is<br />
an overwhelming case to justify the development<br />
of human papillomavirus (HPV)<br />
vaccines. At least 50% of sexually active<br />
adults have had a genital HPV infection. Socalled<br />
“low-risk” HPV types cause benign<br />
lesions or genital warts, while others, called<br />
“high-risk” or “oncogenic” types, are the<br />
principal cause of cervical <strong>cancer</strong> and are<br />
also associated with other <strong>cancer</strong>s of the<br />
anogenital region, and possibly with <strong>cancer</strong>s<br />
of the upper aerodigestive tract and of<br />
the skin (Chronic infections, p56; Cancers<br />
of the female reproductive tract, p215).<br />
Since both genital warts and cervical <strong>cancer</strong><br />
rates are rising in young women in<br />
some populations, the burden of HPV-associated<br />
disease is likely to increase in coming<br />
decades.<br />
HPV-associated lesions can regress spontaneously<br />
due to cell-mediated immunity.<br />
This is indicated by an increased risk of<br />
HPV infection and of HPV-associated<br />
lesions in immunosuppressed patients, and<br />
the observation that neutralizing antibodies<br />
can block HPV infection in vivo and in vitro<br />
[1]. On the other hand, the occurrence of<br />
chronic HPV infections and reinfections<br />
suggests that in some individuals natural<br />
immunity is not effective in controlling HPV<br />
148 Prevention and screening<br />
infection. Although the exact mechanisms<br />
of immune evasion are not fully understood,<br />
the development of effective vaccines<br />
for papillomaviruses in various animal<br />
models [2-4] has stimulated the development<br />
of similar vaccines for humans.<br />
Three main types of HPV vaccine are being<br />
developed: prophylactic vaccines, therapeutic<br />
vaccines and combined or chimeric<br />
vaccines which have both effects.<br />
Prophylactic vaccines<br />
Vaccines based on the induction of neutralizing<br />
antibodies against the HPV structural<br />
proteins L1 and L2 are termed “prophylactic”.<br />
The generation of virus-like<br />
particles (VLPs), which are morphologically<br />
indistinguishable from authentic virions,<br />
apart from lacking the viral genome [5],<br />
has greatly accelerated the development<br />
of these vaccines (Table 4.9).<br />
VLPs for HPV 6, 11, 16, 18, 31, 33, 39, 45<br />
and 58 have been produced in various laboratories.<br />
At least five HPV VLP-based<br />
vaccines have been developed and have<br />
gone through pre-clinical evaluation.<br />
Phase I-II clinical trials to assess safety,<br />
immunogenicity, dose, schedule, route of<br />
administration and adjuvants for these<br />
vaccines are being planned or have been<br />
initiated. The US National Cancer Institute<br />
(NCI) vaccine has been shown, in a phase<br />
I study of 58 women and 14 men, to be<br />
able to induce serum antibody titres that<br />
are approximately 40-fold higher than that<br />
observed during natural infection [6].<br />
However, certain basic issues need to be<br />
solved before the mass use of these vaccines<br />
can commence [7,8]. Such issues<br />
include the HPV types to be included (HPV<br />
16 accounts for 50% and HPV 18, 31 and<br />
45 for a further 30% of cervical <strong>cancer</strong>s),<br />
the route of administration, the target<br />
population (ideally infants) and the cost<br />
(Table 4.10). The ideal HPV vaccine will<br />
have to be polyvalent (i.e. contain the<br />
VLPs of the HPV types most commonly<br />
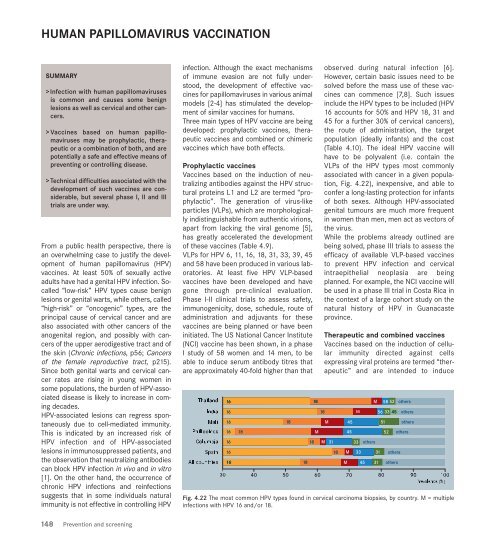
associated with <strong>cancer</strong> in a given population,<br />
Fig. 4.22), inexpensive, and able to<br />
confer a long-lasting protection for infants<br />
of both sexes. Although HPV-associated<br />
genital tumours are much more frequent<br />
in women than men, men act as vectors of<br />
the virus.<br />
While the problems already outlined are<br />
being solved, phase III trials to assess the<br />
efficacy of available VLP-based vaccines<br />
to prevent HPV infection and cervical<br />
intraepithelial neoplasia are being<br />
planned. For example, the NCI vaccine will<br />
be used in a phase III trial in Costa Rica in<br />
the context of a large cohort study on the<br />
natural history of HPV in Guanacaste<br />
province.<br />
Therapeutic and combined vaccines<br />
Vaccines based on the induction of cellular<br />
immunity directed against cells<br />
expressing viral proteins are termed “therapeutic”<br />
and are intended to induce<br />
Fig. 4.22 The most common HPV types found in cervical carcinoma biopsies, by country. M = multiple<br />
infections with HPV 16 and/or 18.