Phase Transfer Catalysis - Publications of the IAS Fellows
Phase Transfer Catalysis - Publications of the IAS Fellows
Phase Transfer Catalysis - Publications of the IAS Fellows
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
_~~~~~~~~~~~~~~~~~~~~<br />
I<br />
1<br />
I<br />
I<br />
1<br />
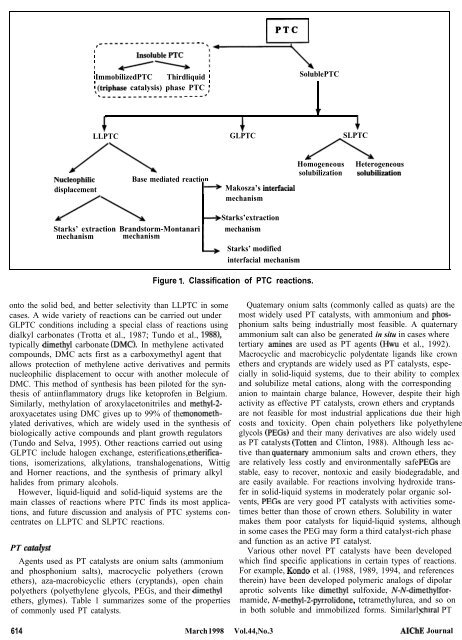
rl PTC<br />
b$pT y-\<br />
; Immobilized PTC Third liquid : Soluble PTC<br />
\(triphase catalysis) phase PTC ;<br />
.~~~~~~~.m~~~~~~~~ __-..e<br />
LLPTC GLPTC SLPTC<br />
/\<br />
Nucleophihc Base mediated reaction<br />
displacement P Makosza’s intetfacial<br />
mechanism<br />
4 Starks’ extraction<br />
Starks’ extraction Brandstorm-Montanari mechanism<br />
mechanism mechanism<br />
L<br />
onto <strong>the</strong> solid bed, and better selectivity than LLPTC in some<br />
cases. A wide variety <strong>of</strong> reactions can be carried out under<br />
GLPTC conditions including a special class <strong>of</strong> reactions using<br />
dialkyl carbonates (Trotta et al., 1987; Tundo et al., 1988),<br />
typically dimethyl carbonate (DMC). In methylene activated<br />
compounds, DMC acts first as a carboxymethyl agent that<br />
allows protection <strong>of</strong> methylene active derivatives and permits<br />
nucleophilic displacement to occur with ano<strong>the</strong>r molecule <strong>of</strong><br />
DMC. This method <strong>of</strong> syn<strong>the</strong>sis has been piloted for <strong>the</strong> syn<strong>the</strong>sis<br />
<strong>of</strong> antiinflammatory drugs like ketopr<strong>of</strong>en in Belgium.<br />
Similarly, methylation <strong>of</strong> aroxylacetonitriles and methyl-2aroxyacetates<br />
using DMC gives up to 99% <strong>of</strong> <strong>the</strong> monomethylated<br />
derivatives, which are widely used in <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong><br />
biologically active compounds and plant growth regulators<br />
(Tundo and Selva, 1995). O<strong>the</strong>r reactions carried out using<br />
GLPTC include halogen exchange, esterifications, e<strong>the</strong>rifications,<br />
isomerizations, alkylations, transhalogenations, Wittig<br />
and Horner reactions, and <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong> primary alkyl<br />
halides from primary alcohols.<br />
However, liquid-liquid and solid-liquid systems are <strong>the</strong><br />
main classes <strong>of</strong> reactions where PTC finds its most applications,<br />
and future discussion and analysis <strong>of</strong> PTC systems concentrates<br />
on LLPTC and SLPTC reactions.<br />
PT catabst<br />
Agents used as PT catalysts are onium salts (ammonium<br />
and phosphonium salts), macrocyclic polye<strong>the</strong>rs (crown<br />
e<strong>the</strong>rs), aza-macrobicyclic e<strong>the</strong>rs (cryptands), open chain<br />
polye<strong>the</strong>rs (polyethylene glycols, PEGS, and <strong>the</strong>ir dimethyl<br />
e<strong>the</strong>rs, glymes). Table 1 summarizes some <strong>of</strong> <strong>the</strong> properties<br />
<strong>of</strong> commonly used PT catalysts.<br />
Starks’ modified<br />
interfacial mechanism<br />
Figure 1. Classification <strong>of</strong> PTC reactions.<br />
Homogeneous Heterogeneous<br />
solubilization solubihxation<br />
Quatemary onium salts (commonly called as quats) are <strong>the</strong><br />
most widely used PT catalysts, with ammonium and phosphonium<br />
salts being industrially most feasible. A quaternary<br />
ammonium salt can also be generated in situ in cases where<br />
tertiary amines are used as PT agents (Hwu et al., 1992).<br />
Macrocyclic and macrobicyclic polydentate ligands like crown<br />
e<strong>the</strong>rs and cryptands are widely used as PT catalysts, especially<br />
in solid-liquid systems, due to <strong>the</strong>ir ability to complex<br />
and solubilize metal cations, along with <strong>the</strong> corresponding<br />
anion to maintain charge balance, However, despite <strong>the</strong>ir high<br />
activity as effective PT catalysts, crown e<strong>the</strong>rs and cryptands<br />
are not feasible for most industrial applications due <strong>the</strong>ir high<br />
costs and toxicity. Open chain polye<strong>the</strong>rs like polyethylene<br />
glycols (PEGS) and <strong>the</strong>ir many derivatives are also widely used<br />
as PT catalysts (Totten and Clinton, 1988). Although less active<br />
than quaternary ammonium salts and crown e<strong>the</strong>rs, <strong>the</strong>y<br />
are relatively less costly and environmentally safe. PEGS are<br />
stable, easy to recover, nontoxic and easily biodegradable, and<br />
are easily available. For reactions involving hydroxide transfer<br />
in solid-liquid systems in moderately polar organic solvents,<br />
PEGS are very good PT catalysts with activities sometimes<br />
better than those <strong>of</strong> crown e<strong>the</strong>rs. Solubility in water<br />
makes <strong>the</strong>m poor catalysts for liquid-liquid systems, although<br />
in some cases <strong>the</strong> PEG may form a third catalyst-rich phase<br />
and function as an active PT catalyst.<br />
Various o<strong>the</strong>r novel PT catalysts have been developed<br />
which find specific applications in certain types <strong>of</strong> reactions.<br />
For example, Kondo et al. (1988, 1989, 1994, and references<br />
<strong>the</strong>rein) have been developed polymeric analogs <strong>of</strong> dipolar<br />
aprotic solvents like dimethyl sulfoxide, N-N-dimethylformamide,<br />
N-methyl-2-pyrrolidone, tetramethylurea, and so on<br />
in both soluble and immobilized forms. Similarly, chiral PT<br />
614 March 1998 Vol. 44, No. 3 AIChE Journal