Silyl Ethers - Thieme Chemistry
Silyl Ethers - Thieme Chemistry
Silyl Ethers - Thieme Chemistry
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
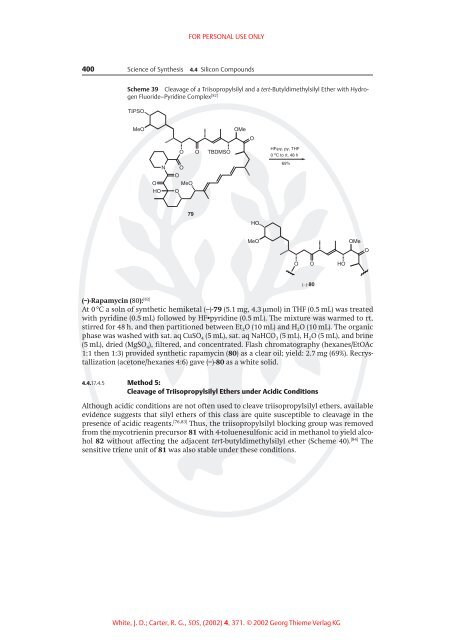
Scheme 39 Cleavage of a Triisopropylsilyl and a tert-Butyldimethylsilyl Ether with Hydrogen<br />
Fluoride±Pyridine Complex [82]<br />
TIPSO<br />
MeO<br />
O<br />
HO<br />
N<br />
O<br />
O<br />
O<br />
MeO<br />
O<br />
FOR PERSONAL USE ONLY<br />
400 Science of Synthesis 4.4 Silicon Compounds<br />
79<br />
O<br />
TBDMSO<br />
OMe<br />
O<br />
HO<br />
MeO<br />
HF py, py, THF<br />
0 oC to rt, 48 h<br />
69%<br />
O O HO<br />
(±)-Rapamycin (80): [82]<br />
At 0 8C a soln of synthetic hemiketal (±)-79 (5.1 mg, 4.3 ìmol) in THF (0.5 mL) was treated<br />
with pyridine (0.5 mL) followed by HF·pyridine (0.5 mL). The mixture was warmed to rt,<br />
stirred for 48 h, and then partitioned between Et 2O (10 mL) and H 2O (10 mL). The organic<br />
phase was washed with sat. aq CuSO 4 (5 mL), sat. aq NaHCO 3 (5 mL), H 2O (5 mL), and brine<br />
(5 mL), dried (MgSO 4), filtered, and concentrated. Flash chromatography (hexanes/EtOAc<br />
1:1 then 1:3) provided synthetic rapamycin (80) as a clear oil; yield: 2.7 mg (69%). Recrystallization<br />
(acetone/hexanes 4:6) gave (±)-80 as a white solid.<br />
(−)-80<br />
4.4.17.4.5 Method 5:<br />
Cleavage ofTriisopropylsilyl <strong>Ethers</strong> under Acidic Conditions<br />
Although acidic conditions are not often used to cleave triisopropylsilyl ethers, available<br />
evidence suggests that silyl ethers of this class are quite susceptible to cleavage in the<br />
presence of acidic reagents. [76,83] Thus, the triisopropylsilyl blocking group was removed<br />
from the mycotrienin precursor 81 with 4-toluenesulfonic acid in methanol to yield alcohol<br />
82 without affecting the adjacent tert-butyldimethylsilyl ether (Scheme 40). [84] The<br />
sensitive triene unit of 81 was also stable under these conditions.<br />
White, J. D.; Carter, R. G., SOS, (2002) 4, 371. 2002 Georg <strong>Thieme</strong> Verlag KG<br />
OMe<br />
O