Silyl Ethers - Thieme Chemistry
Silyl Ethers - Thieme Chemistry
Silyl Ethers - Thieme Chemistry
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
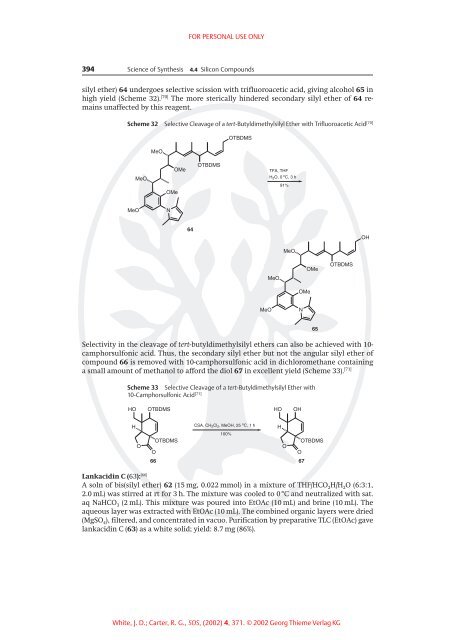
silyl ether) 64 undergoes selective scission with trifluoroacetic acid, giving alcohol 65 in<br />
high yield (Scheme 32). [70] The more sterically hindered secondary silyl ether of 64 remains<br />
unaffected by this reagent.<br />
Scheme 32 Selective Cleavage of a tert-Butyldimethylsilyl Ether with Trifluoroacetic Acid [70]<br />
MeO<br />
MeO<br />
MeO N<br />
OMe<br />
OMe<br />
64<br />
OTBDMS<br />
OTBDMS<br />
TFA, THF<br />
H2O, 0 oC, 3 h<br />
91%<br />
MeO<br />
MeO<br />
MeO N<br />
OMe<br />
OMe<br />
65<br />
OTBDMS<br />
Selectivity in the cleavage of tert-butyldimethylsilyl ethers can also be achieved with 10camphorsulfonic<br />
acid. Thus, the secondary silyl ether but not the angular silyl ether of<br />
compound 66 is removed with 10-camphorsulfonic acid in dichloromethane containing<br />
a small amount of methanol to afford the diol 67 in excellent yield (Scheme 33). [71]<br />
Scheme 33 Selective Cleavage of a tert-Butyldimethylsilyl Ether with<br />
10-Camphorsulfonic Acid [71]<br />
HO<br />
H<br />
O<br />
OTBDMS<br />
O<br />
OTBDMS<br />
FOR PERSONAL USE ONLY<br />
394 Science of Synthesis 4.4 Silicon Compounds<br />
CSA, CH2Cl2, MeOH, 25 oC, 1 h<br />
100%<br />
HO<br />
66 67<br />
H<br />
O<br />
OH<br />
O<br />
OTBDMS<br />
Lankacidin C (63): [66]<br />
A soln of bis(silyl ether) 62 (15 mg, 0.022 mmol) in a mixture of THF/HCO 2H/H 2O (6:3:1,<br />
2.0 mL) was stirred at rt for 3 h. The mixture was cooled to 08C and neutralized with sat.<br />
aq NaHCO 3 (2 mL). This mixture was poured into EtOAc (10 mL) and brine (10 mL). The<br />
aqueous layer was extracted with EtOAc (10 mL). The combined organic layers were dried<br />
(MgSO 4), filtered, and concentrated in vacuo. Purification by preparative TLC (EtOAc) gave<br />
lankacidin C (63) as a white solid; yield: 8.7 mg (86%).<br />
White, J. D.; Carter, R. G., SOS, (2002) 4, 371. 2002 Georg <strong>Thieme</strong> Verlag KG<br />
OH