Kinetic Resolutions - The Stoltz Group
Kinetic Resolutions - The Stoltz Group
Kinetic Resolutions - The Stoltz Group
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Requirements<br />
- Enantioselective reaction (some chiral influence)<br />
- Significant difference in reaction rate<br />
- Incomplete conversion to product<br />
Preferable<br />
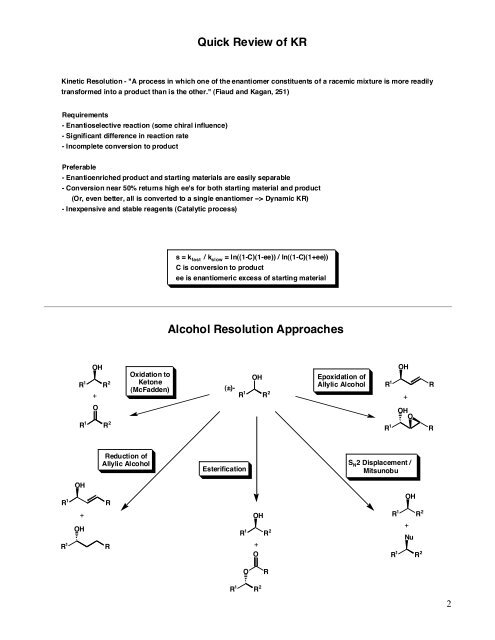
Quick Review of KR<br />
<strong>Kinetic</strong> Resolution - "A process in which one of the enantiomer constituents of a racemic mixture is more readily<br />
transformed into a product than is the other." (Fiaud and Kagan, 251)<br />
- Enantioenriched product and starting materials are easily separable<br />
- Conversion near 50% returns high ee's for both starting material and product<br />
(Or, even better, all is converted to a single enantiomer --> Dynamic KR)<br />
- Inexpensive and stable reagents (Catalytic process)<br />
R 1<br />
R 1<br />
R 1<br />
R 1<br />
OH<br />
+<br />
OH<br />
OH<br />
+<br />
O<br />
R 2<br />
R 2<br />
Oxidation to<br />
Ketone<br />
(McFadden)<br />
Reduction of<br />
Allylic Alcohol<br />
R<br />
R<br />
s = k fast / k slow = ln((1-C)(1-ee)) / ln((1-C)(1+ee))<br />
C is conversion to product<br />
ee is enantiomeric excess of starting material<br />
Alcohol Resolution Approaches<br />
(±)-<br />
R 1<br />
Esterification<br />
R 1<br />
R 1<br />
O<br />
OH<br />
OH<br />
+<br />
O<br />
R 2<br />
R 2<br />
R 2<br />
R<br />
Epoxidation of<br />
Allylic Alcohol<br />
R 1<br />
R 1<br />
S N 2 Displacement /<br />
Mitsunobu<br />
R 1<br />
R 1<br />
OH<br />
+<br />
OH<br />
O<br />
OH<br />
+<br />
Nu<br />
R 2<br />
R 2<br />
R<br />
R<br />
2