Automation and Real-time PCR Products and ... - Eppendorf AG
Automation and Real-time PCR Products and ... - Eppendorf AG
Automation and Real-time PCR Products and ... - Eppendorf AG
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Automation</strong> <strong>and</strong> <strong>Real</strong>-<strong>time</strong> <strong>PCR</strong><br />
<strong>Products</strong> <strong>and</strong> Applications 2008/09
2<br />
What ARTS can do for you.<br />
<strong>Eppendorf</strong> ARTS st<strong>and</strong>s for “<strong>Automation</strong> <strong>and</strong> <strong>Real</strong>-<strong>time</strong> Systems,”<br />
<strong>and</strong> it is a special division of <strong>Eppendorf</strong> North America with its own<br />
sales <strong>and</strong> support team. They focus solely on the intricacies of real-<br />
<strong>time</strong> <strong>PCR</strong> <strong>and</strong> liquid h<strong>and</strong>ling automation, so you can trust them to<br />
underst<strong>and</strong> your applications <strong>and</strong> recommend the right <strong>Eppendorf</strong><br />
system to meet your exact needs.<br />
Combining quality products with expert, reliable support.<br />
Go to virtually any lab anywhere in the world, <strong>and</strong> you will see<br />
<strong>Eppendorf</strong> products hard at work—it’s a sure sign of those<br />
labs’ strong commitment to the quality of their research <strong>and</strong><br />
the accuracy of their results. Why? Because <strong>Eppendorf</strong> has a<br />
reputation for producing the best <strong>and</strong> most reliable laboratory<br />
equipment <strong>and</strong> consumables—dating back to the 1950s <strong>and</strong> ‘60s,<br />
when we introduced our flagship products: pipettes, centrifuges,<br />
tips <strong>and</strong> microcentrifuge tubes. Fast-forward to a new century—<strong>and</strong><br />
a new era in liquid h<strong>and</strong>ling <strong>and</strong> scientific research—<strong>and</strong> see our<br />
innovative solutions for automated pipetting <strong>and</strong> <strong>PCR</strong> applications.<br />
<strong>Eppendorf</strong> ARTS <br />
Specializing in automation <strong>and</strong> real-<strong>time</strong> systems.<br />
Just one look at our epMotion ® automated pipetting systems,<br />
<strong>and</strong> you will love pipetting—it’s true! Say goodbye to tedium <strong>and</strong><br />
repetitive stress injuries, as well as compromised results due<br />
to manual pipetting error. Say hello to h<strong>and</strong>s-free pipetting with<br />
complete accuracy <strong>and</strong> reproducibility. epMotion lets you get<br />
to more important <strong>and</strong> interesting work—<strong>and</strong> have more <strong>time</strong> to<br />
concentrate on it. And we’ve priced it so that every lab can afford<br />
to upgrade. Complete application <strong>and</strong> product details begin<br />
on page 6.<br />
<strong>Automation</strong> is only one half of the ARTS story. Take a look at our<br />
space-saving Mastercycler ® ep realplex q<strong>PCR</strong> instruments: they’re<br />
designed with the most advanced optics <strong>and</strong> thermal blocks to<br />
satisfy even the most stringent q<strong>PCR</strong> requirements <strong>and</strong> give you<br />
highly sensitive <strong>and</strong> accurate results. Our ultra-fast <strong>and</strong> flexible<br />
models have also been documented to cut experiment <strong>time</strong> in half<br />
when compared with older, slower systems in use today. Read all<br />
about them beginning on page 29.<br />
<strong>Eppendorf</strong> ARTS is not just about products; we’re equally<br />
passionate about the type of personal service that comes<br />
along with them. Enjoy the service <strong>and</strong> support of dedicated<br />
professionals to assist you every step of the way with your system<br />
purchase <strong>and</strong> with after-sale applications support <strong>and</strong> service.
Meet your ARTS team.<br />
They are your colleagues in science <strong>and</strong> experts in application<br />
support <strong>and</strong> customer relations, whose singular purpose is to<br />
provide you with the best research tools <strong>and</strong> support available<br />
anywhere in the United States <strong>and</strong> Canada.<br />
Count on your local ARTS Specialist, a highly trained scientist with<br />
extensive bench experience, to assess your needs <strong>and</strong> recommend<br />
the <strong>Eppendorf</strong> system that’s best for you <strong>and</strong> your research.<br />
Then meet your local Field Service Engineer, who will install your<br />
system <strong>and</strong> train you to a high level of user confidence. Call our<br />
Customer Support Representatives with your general inquiries, <strong>and</strong><br />
for method-related questions you can always call our dedicated<br />
applications hotline for a direct response from a staff scientist.<br />
Take advantage of everything ARTS has to offer.<br />
Enjoy innovative products that answer to your needs <strong>and</strong> take<br />
your research to the next level.<br />
Enjoy the service <strong>and</strong> support of dedicated professionals to<br />
assist you every step of the way with your system purchase <strong>and</strong><br />
application support.<br />
Enjoy the peace of mind that our Performance Plans bring,<br />
keeping your instruments operating at peak efficiency for years to<br />
come. Services are performed on site by a dedicated <strong>Eppendorf</strong><br />
service professional to keep down<strong>time</strong> at a minimum.<br />
We also have several service centers located across the United<br />
States <strong>and</strong> Canada to provide expert validation, calibration, <strong>and</strong><br />
a host of other repair services, should the need arise.<br />
<strong>Eppendorf</strong>: In touch with life, in touch with you.<br />
“In touch with life” is not just a catch-phrase under our logo—it’s<br />
our mission <strong>and</strong> has been for over 60 years. By introducing cutting-<br />
edge products <strong>and</strong> technologies, we support the advancement<br />
of life science research; <strong>and</strong> through our ARTS organization we<br />
support YOU in making these advancements possible.<br />
Your ARTS team is here for you:<br />
Contact us by phone<br />
1-800-645-3050, M–F 8:30 am – 6:00 pm EST (U.S)<br />
1-800-263-8715, M–F 8:30 am – 5:00 pm EST (Canada)<br />
Contact us by email<br />
artsinfo@eppendorf.com (general inquiries)<br />
arts@eppendorf.com (applications support)<br />
Visit us online<br />
In the U.S., www.eppendorfna.com<br />
In Canada, www.eppendorf.ca
Table of contents<br />
Info<br />
Table of contents<br />
Introduction to epMotion . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6<br />
epMotion selection guide . . . . . . . . . . . . . . . . . . . . . . . . . . . . .7<br />
epMotion system overview . . . . . . . . . . . . . . . . . . . . . . . . . . . .8<br />
Automated real-<strong>time</strong> <strong>PCR</strong> set-up . . . . . . . . . . . . . . . . . . . . . .10<br />
Automated nucleic acid preparation . . . . . . . . . . . . . . . . . . . .11<br />
epMotion 5070 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .12<br />
epMotion 5075 LH . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14<br />
epMotion 5075 VAC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .16<br />
epMotion 5075 MC . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .18<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .29<br />
Mastercycler ep realplex. . . . . . . . . . . . . . . . . . . . . . . . . . . . . .30<br />
Gradient function: a highly useful tool for<br />
optimizing real-<strong>time</strong> <strong>PCR</strong> . . . . . . . . . . . . . . . . . . . . . . . . . . . . .34<br />
Impulse <strong>PCR</strong>: a novel, accelerated <strong>and</strong><br />
improved q<strong>PCR</strong> Hot Start method . . . . . . . . . . . . . . . . . . . . . .36<br />
High-speed, real-<strong>time</strong> <strong>PCR</strong> assay design<br />
for realplex silver block models . . . . . . . . . . . . . . . . . . . . . . . .38<br />
Automated genomic DNA purification<br />
from tissue culture cells . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .49<br />
Isolation of high quality BAC DNA . . . . . . . . . . . . . . . . . . . . . .52<br />
Accurate <strong>and</strong> precise pipetting of soluents . . . . . . . . . . . . . . .56<br />
PureLink 96 Total RNA Purification Kit . . . . . . . . . . . . . . . . .58<br />
Automated genomic DNA purification in<br />
96-well plate <strong>and</strong> 8-well strip format . . . . . . . . . . . . . . . . . . . .60<br />
Mastercycler ep realplex—a flexible device<br />
for fast <strong>and</strong> accurate real-<strong>time</strong> <strong>PCR</strong>. . . . . . . . . . . . . . . . . . . . .65<br />
Automated Pipetting Systems<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong><br />
Applications<br />
Appendix<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
epMotion 5070 CB . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .19<br />
epMotion control options . . . . . . . . . . . . . . . . . . . . . . . . . . . . .20<br />
epBlue PC software for epMotion . . . . . . . . . . . . . . . . . . . .21<br />
epMotion accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .22<br />
epMotion tools <strong>and</strong> tips . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .23<br />
Technical specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . .24<br />
Order information, general . . . . . . . . . . . . . . . . . . . . . . . . . . .25<br />
Order information, accessories . . . . . . . . . . . . . . . . . . . . . . . .26<br />
Benefits of realplex’s homogeneity <strong>and</strong><br />
accuracy on reproducibility in real-<strong>time</strong> q<strong>PCR</strong>. . . . . . . . . . . . .42<br />
Optical concept highlight – 96 well excitation . . . . . . . . . . . . .43<br />
twin.tec <strong>PCR</strong> plates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44<br />
twin.tec real-<strong>time</strong> <strong>PCR</strong> plates. . . . . . . . . . . . . . . . . . . . . . . . . .45<br />
Heat sealing instruments <strong>and</strong> consumables . . . . . . . . . . . . . .46<br />
Low volume real-<strong>time</strong> <strong>PCR</strong><br />
on the Mastercycler ep realplex. . . . . . . . . . . . . . . . . . . . . . . . . 71<br />
<strong>Real</strong>-<strong>time</strong> RT-<strong>PCR</strong> diagnosis of the avian influenza<br />
virus using Mastercycler ® ep realplex S <strong>and</strong><br />
AIV RT-<strong>PCR</strong> kits . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .74<br />
Improved reproducibility <strong>and</strong> sensitivity in real-<strong>time</strong><br />
<strong>PCR</strong> with <strong>Eppendorf</strong> ® twin.tec real-<strong>time</strong> <strong>PCR</strong> plates . . . . . . . .77<br />
Successful q<strong>PCR</strong> with small reaction volumes on<br />
<strong>Eppendorf</strong> Mastercycler ® ep realplex. . . . . . . . . . . . . . . . . . . .80<br />
q<strong>PCR</strong>: Definitions, concepts <strong>and</strong> overview . . . . . . . . . . . . . . .87 Calculating primer quantity . . . . . . . . . . . . . . . . . . . . . . . . . . .93<br />
Detection chemistries. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .88 <strong>PCR</strong> calculator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .94<br />
Methods of real-<strong>time</strong> <strong>PCR</strong> quantification. . . . . . . . . . . . . . . . .90 Abbreviations, symbols <strong>and</strong> conversion factors. . . . . . . . . . . .96<br />
Methods of primer <strong>and</strong> probe validation . . . . . . . . . . . . . . . . .91 Genetic code <strong>and</strong> amino acid properties. . . . . . . . . . . . . . . . .98<br />
References, resources <strong>and</strong> useful websites. . . . . . . . . . . . . . .92<br />
How to buy<br />
<strong>Products</strong> listed in this catalog can be purchased directly from<br />
<strong>Eppendorf</strong> North America. Your local <strong>Eppendorf</strong> ARTS Specialist<br />
can help you choose the <strong>Eppendorf</strong> system that best meets your<br />
needs <strong>and</strong> provide you with a price quotation. When you’re ready<br />
to order, call <strong>Eppendorf</strong> Customer Support at 1-800-645-3050,<br />
ext. 2101. Select products can also be purchased through the<br />
<strong>Eppendorf</strong> eShop at www.eppendorfna.com/eshop.<br />
Orders can be placed using a purchase order or major credit card.<br />
We accept VISA ® , MasterCard ® <strong>and</strong> American Express ® . We also<br />
accept EDI order transfers. Please contact our Customer Support<br />
department for more information.<br />
Prices <strong>and</strong> specifications<br />
Product appearance, specifications <strong>and</strong>/or prices are subject<br />
to change without notice.<br />
Product warranty<br />
<strong>Eppendorf</strong> products are warranted against defects in workmanship<br />
<strong>and</strong> materials. Contact our Customer Support department at<br />
1-800-645-3050, ext. 2101, for warranty information on specific<br />
products <strong>and</strong> warranty extensions that may be available. Product<br />
warranty information is also available on our website.<br />
Freight terms<br />
Parcel <strong>and</strong> motor freight shipments ordered collect are F.O.B.<br />
Westbury, NY. In most cases, prepaid shipments are F.O.B.<br />
destination.
Automated Pipetting<br />
Systems<br />
ARTS
Instruments | Automated Pipetting<br />
Automated pipetting<br />
epMotion Automated Pipetting Systems: the next step in liquid h<strong>and</strong>ling<br />
Ever since <strong>Eppendorf</strong> invented the microliter pipette 45 years ago,<br />
we have been passionate about precision in liquid h<strong>and</strong>ling. This<br />
passion for excellence is clearly demonstrated in our epMotion ®<br />
systems. These products are flexible enough to use in virtually any<br />
assay that can be manually pipetted, from sample prep to assay<br />
set-up.<br />
With epMotion automated systems, you will see pipetting in a new<br />
light. All your routine pipetting tasks, whether small or large, will be<br />
automated with more precision <strong>and</strong> reproducibility than you ever<br />
experienced with manual pipetting.<br />
Learn how simple it is to automate. The revolutionary <strong>and</strong> easy<br />
to use epMotion lends itself to all laboratory environments. The<br />
software is so intuitive you can learn it in just one morning <strong>and</strong> be<br />
up <strong>and</strong> running protocols that same afternoon. Options for the<br />
integrated compact control panel or the flexible PC version make<br />
it easy for anyone to use, anywhere. Features such as pipetting<br />
pattern recognition or the ready to go Plug’n’Prep ® methods ensure<br />
that entering the world of automation is fast <strong>and</strong> easy.<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Learn about our unique pipetting range from 1 µl to 1,000 µl. With<br />
epMotion, you can experience utmost precision even at the lowest<br />
volumes without complex pipetting comm<strong>and</strong>s. Our open platform<br />
lets you automate your applications with all your favorite labware.<br />
We have more than 700 single-tube formats, well plates, <strong>and</strong> even<br />
unusual plastics such as real-<strong>time</strong> rotor tubes included in our<br />
database <strong>and</strong> available for immediate use.<br />
Discover new possibilities: Save reagent costs in real-<strong>time</strong><br />
<strong>PCR</strong>, enjoy the choices in nucleic acid preparation, get more<br />
performance out of your cell culture work, finish first in your<br />
genomics projects, <strong>and</strong> experience more reliability in clinical<br />
research. With five models, two instrument sizes, <strong>and</strong> multiple<br />
pipetting deck combinations to choose from, epMotion is the<br />
perfect companion for your workload. epMotion —discover<br />
the possibilities.
epMotion selection guide<br />
epMotion Capacity Applications<br />
0 0 4-position deck <strong>and</strong><br />
3 virtual positions<br />
System description page 12<br />
0 0 CB 4-position deck <strong>and</strong><br />
3 virtual positions,<br />
specially designed to<br />
fit into a laminar flow<br />
hood or fume hood<br />
System description page 19<br />
0 LH 12-position deck<br />
System description page 14<br />
‡ Transporting<br />
‡ Stacking ability<br />
‡ 3 thermo modules<br />
(optional)<br />
0 VAC 11-position deck <strong>and</strong><br />
1 vacuum station<br />
System description page 1<br />
0 MC 9-position deck<br />
<strong>and</strong> 1 position<br />
for Mastercycler ® ep<br />
System description page 18<br />
Read our detailed epMotion application examples:<br />
Automated real-<strong>time</strong> <strong>PCR</strong> set-up, page 10<br />
Automated nucleic acid preparation, page 11<br />
General pipetting tasks<br />
‡ Serial dilutions<br />
‡ Tube to plate transfer<br />
‡ Reformatting of plates<br />
‡ Concentration normalization<br />
‡ Hit-picking<br />
‡ Reagent transfer<br />
‡ Pooling<br />
Examples: All routine pipetting, e.g. sample/<br />
reagent transfer, q<strong>PCR</strong> or immunoassays<br />
Cell culture<br />
‡ Cell separation<br />
‡ Change of media<br />
‡ Cytotoxicity assays<br />
‡ Growth curves<br />
‡ Soft agar assays<br />
‡ Cell-based assays<br />
‡ Apoptosis assays<br />
Examples: Cell culture maintenance<br />
<strong>and</strong> assays<br />
Genomics applications<br />
‡ <strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> set-up<br />
‡ <strong>PCR</strong> set-up with automated amplification<br />
‡ Sequencing set-up<br />
‡ SNP detection<br />
‡ Forensics<br />
Examples: All routine pipetting, e.g. sample/<br />
reagent transfer, q<strong>PCR</strong> or immunoassays<br />
Nucleic acid purification<br />
‡ gDNA from various matrixes<br />
‡ Total <strong>and</strong> viral RNA<br />
‡ Plasmid preparation<br />
‡ BAC purification<br />
‡ <strong>PCR</strong> <strong>and</strong> sequencing clean-up<br />
‡ Bacterial DNA<br />
Automated pipetting<br />
Examples: Nucleic acid extraction, Plug’n’Prep ®<br />
protocols, filtration, <strong>and</strong> solid phase extraction<br />
Complex assays<br />
‡ Luminex ® assay set-up<br />
‡ HLA genotyping<br />
‡ Advalytix <strong>PCR</strong> slide pipetting<br />
Examples: Fully automated <strong>PCR</strong>: set-up<br />
<strong>and</strong> amplification<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
Automated Pipetting | Instruments
Instruments | Automated Pipetting<br />
8<br />
Automated pipetting<br />
epMotion Automated Pipetting Systems<br />
Easy to use<br />
‡ No other automation compares to epMotion<br />
– Software can be fully mastered in only a few hours<br />
– Optical sensor for safe operation examines<br />
the worktable for correct loading<br />
– No teaching required – labware data is available<br />
as free download at www.epMotion.com<br />
Accurate<br />
‡ Superior automated liquid h<strong>and</strong>ling<br />
– Pipetting tools with single- <strong>and</strong> eight-channel<br />
<strong>Eppendorf</strong> air cushion technology<br />
– Pipetting range from 1 to 1,000 µl, always<br />
from air to minimize cross-contamination<br />
– Typical pipetting precision below 2% CV at 1 µl*<br />
* <strong>Eppendorf</strong> application note 168: “Measuring the Accuracy <strong>and</strong> Precision of the<br />
epMotion ® 5070 Workstation Using the Artel Multichannel Verification System (MVS ® ).”<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
Flexible<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
‡ Every application, every day<br />
– Tubes range from 0.2 ml <strong>PCR</strong> to 50 ml blue-cap tubes<br />
– Plates range from 6 to 96 <strong>and</strong> 384 wells<br />
– Filter <strong>and</strong> non-filter tips, specially selected for straightness,<br />
non-carbonized to reduce costs<br />
– Sterile tips available<br />
epMotion application overview, see page .<br />
To learn more visit www.epmotion.com.
epMotion Automated Pipetting Systems<br />
Five different epMotion systems give you the freedom to<br />
h<strong>and</strong>le any application:<br />
‡ epMotion 5070<br />
‡ epMotion 5070 CB (cell biology)<br />
‡ epMotion 5075 LH (liquid h<strong>and</strong>ling)<br />
‡ epMotion 5075 VAC (vacuum technology)<br />
‡ epMotion 5075 MC (<strong>PCR</strong> set-up <strong>and</strong> amplification)<br />
epMotion selection guide, see page .<br />
Control options for all models<br />
‡ Compact epMotion control panel (see page 20)<br />
‡ Easily operated epMotion PC version (see page 20)<br />
Features for all models<br />
Automated pipetting<br />
‡ Verified single-channel <strong>and</strong> multi-channel pipetting tools<br />
‡ Completely contained housing including door safety mechanism<br />
‡ Optical sensor for identification of labware, tips, <strong>and</strong><br />
reagent volumes<br />
‡ Easy to use software with pre-loaded labware files<br />
Options for 0 models<br />
‡ Plate gripper<br />
‡ Up to three temperature control positions<br />
‡ Built-in vacuum<br />
‡ Built-in thermal cycler<br />
Check out our latest video at www.epmotion.com/video.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
9<br />
Automated Pipetting | Instruments
Instruments | Automated Pipetting<br />
10<br />
Automated pipetting<br />
��� �� ������<br />
Automated real-<strong>time</strong> <strong>PCR</strong> set-up<br />
Less reagents needed, more reproducibility delivered<br />
With epMotion, you can increase reproducibility in real-<strong>time</strong> <strong>PCR</strong><br />
experiments significantly. In an experimental attempt to pipette<br />
a real-<strong>time</strong> <strong>PCR</strong> sample 96 <strong>time</strong>s, the epMotion shows superior<br />
results compared to manual pipetting by reducing the st<strong>and</strong>ard<br />
deviations from 0.34 to 0.18 (Fig. 1).* This decreases the number<br />
of sample replicates needed to statistically confirm an increase in<br />
gene expression. With epMotion, your real-<strong>time</strong> <strong>PCR</strong> experiments<br />
become more significant.<br />
* Data kindly provided by Mikael Kubista, TATAA Biocenter, Göteborg, Sweden.<br />
��<br />
��<br />
��<br />
��<br />
��<br />
��<br />
�<br />
�<br />
�<br />
�<br />
Visit www.save-reagents.com<br />
����<br />
����<br />
����<br />
����<br />
����<br />
����<br />
����<br />
����<br />
����<br />
����<br />
����<br />
����<br />
�<br />
���<br />
���<br />
���<br />
���<br />
���<br />
���<br />
���<br />
���<br />
���<br />
���<br />
���<br />
���<br />
� � � �� � �<br />
‡ Fig. 1a/1b: Reproducibility in real-<strong>time</strong> <strong>PCR</strong> set-up<br />
left: Manual dispensing (SD=0.34), right: Automated dispensing (SD=0.18)*<br />
‡ With epMotion, your<br />
real-<strong>time</strong> <strong>PCR</strong> cycler<br />
becomes ultra-efficient.<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Versatility: Enjoy it with any real-<strong>time</strong> system!<br />
The epMotion is compatible with any real-<strong>time</strong> cycler.<br />
With epMotion, you have the flexibility of using single tubes,<br />
96- or 384-well plates, or unusual plastics such as real-<strong>time</strong> rotor<br />
tube-strips. You can even save on tips with the multi-dispensing<br />
capability of epMotion.<br />
epMotion: Spend less on reagents!<br />
Reagents are a critical cost factor when performing real-<strong>time</strong><br />
<strong>PCR</strong> experiments, <strong>and</strong> some<strong>time</strong>s the cost of reagents can even<br />
limit your research projects. epMotion delivers utmost pipetting<br />
precision from 1 µl to 1,000 µl. Reaction volumes as low as 5 µl<br />
can easily be achieved, cutting your reagent costs by as much<br />
as 80%.<br />
��� �� ������<br />
��<br />
��<br />
��<br />
��<br />
��<br />
��<br />
�<br />
�<br />
�<br />
�<br />
����<br />
����<br />
����<br />
����<br />
����<br />
����<br />
����<br />
����<br />
����<br />
����<br />
����<br />
����<br />
�<br />
���<br />
���<br />
���<br />
���<br />
���<br />
���<br />
���<br />
���<br />
���<br />
���<br />
���<br />
���<br />
� � � �� � �
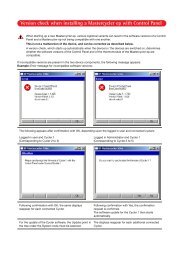
Plug'n'Prep automated nucleic acid preparation<br />
More choices with pre-tested Plug’n’Prep ® protocols<br />
Purification of nucleic acids is a <strong>time</strong>-consuming process. epMotion<br />
provides walk-away automation.<br />
With the unique Plug’n’Prep ® technology, you are only four<br />
steps away from truly simple <strong>and</strong> fast purification of up to<br />
9 samples per run:<br />
1<br />
2<br />
3<br />
4<br />
Finally forget about tedious <strong>and</strong> stressful repetitive<br />
pipetting. Choose your preferred extraction br<strong>and</strong><br />
<strong>and</strong> benefit from manufacturer pre-tested<br />
Plug’n’Prep ® methods.<br />
Download the pre-tested methods<br />
from www.epMotion.com.<br />
Transfer the method data<br />
file to the epMotion.<br />
Place samples in the epMotion<br />
<strong>and</strong> press “start”.<br />
1<br />
2<br />
3<br />
4<br />
Automated pipetting<br />
epMotion is a true open system. It allows you to choose the type<br />
of extraction you need from the br<strong>and</strong>s that you prefer.<br />
And since epMotion can fully automate your protocol, you are<br />
available to do other tasks while your sample is being purified. All<br />
epMotion methods have been optimized <strong>and</strong> tested in cooperation<br />
with the kit manufacturer. Our Plug’n’Prep ® protocols provide<br />
superior nucleic acid yield <strong>and</strong> purity.<br />
epMotion automated pipetting systems<br />
give you freedom of choice:<br />
gDNA extraction from blood,<br />
tissue, <strong>and</strong> cultured cells<br />
Total RNA <strong>and</strong><br />
virus RNA isolation<br />
Plasmid <strong>and</strong><br />
BAC purification<br />
<strong>PCR</strong> <strong>and</strong><br />
sequencing clean-up<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
11<br />
Automated Pipetting | Instruments
Instruments | Automated Pipetting<br />
12<br />
Automated pipetting<br />
epMotion 5070 Automated Pipetting System<br />
The epMotion 5070 is the most compact solution for accurate <strong>and</strong><br />
reproducible automated pipetting. With four deck positions, it can<br />
hold tips, reagent reservoirs, single tubes, tube racks, microplates,<br />
or <strong>PCR</strong> plates with up to 384 wells. Two options are available to<br />
control automated pipetting: epMotion PC version or epMotion<br />
control panel (see page 20).<br />
Safety door<br />
epMotion can be fully closed.<br />
Operation is paused when the door<br />
is opened during a run.<br />
4-position worktable<br />
Perfect for up to 192 samples per run.<br />
Specially designed for applications such as<br />
real-<strong>time</strong> <strong>PCR</strong> or immunoassay set-up,<br />
tube to plate transfer, serial dilutions, or sample<br />
normalization.<br />
Control panel<br />
Compact <strong>and</strong> easy to use,<br />
graphical color display.<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
More reliability—change to the best in pipetting.<br />
Get the optimum in reproducible<br />
pipetting for really reproducible results.<br />
“We have used the epMotion 5070 for more than two years<br />
to set up low-volume reactions for our 48-channel<br />
capillary sequencers. The epMotion enables us to run<br />
ten plates a day at 2 µl reaction volume with maximum<br />
reliance <strong>and</strong> reproducibility.”<br />
Dr. Yogesh S. Shouche,<br />
Scientist ‘E’ (In-Charge DNA Sequencing),<br />
National Centre for Cell Science, Pune, India
epMotion 5070 Automated Pipetting System<br />
Automated pipetting – ultra-compact<br />
This makes the epMotion 5070 a perfect match for any routine<br />
application such as serial dilutions, reagent distribution, sample<br />
transfer from tubes to plates, <strong>and</strong> sample normalization.<br />
Due to its exceptional accuracy, the epMotion 5070 sets the<br />
st<strong>and</strong>ard for automated pipetting <strong>and</strong> real-<strong>time</strong> <strong>PCR</strong> set-up. The<br />
epMotion 5070 enables assay set-up with less reagent volume <strong>and</strong><br />
excellent reproducibility – within <strong>and</strong> even across experiments!<br />
Automated pipetting<br />
More savings—reduce reaction volumes<br />
<strong>and</strong> change to more dense plates<br />
to cut reagent costs.<br />
“The epMotion saved us a significant amount of money in<br />
our real-<strong>time</strong> <strong>PCR</strong> work, as we were able to decrease<br />
to a 5 µl total reaction volume. Additionally, we benefited<br />
from absolute reproducibility in pipetting volumes that<br />
is far better than with manual pipetting. We realized that<br />
our investment in the epMotion was easily recovered in<br />
about one year, based on the reagent savings.”<br />
Elaine R. Mardis, Ph.D.<br />
Associate Professor in Genetics <strong>and</strong><br />
Molecular Microbiology, Director, Technology Development<br />
<strong>and</strong> Co-Director, Genome Sequencing Center,<br />
Washington University School of Medicine,<br />
St. Louis, Missouri, USA<br />
Optical sensor<br />
Checks loading of the worktable for tips<br />
(type <strong>and</strong> number), labware type, <strong>and</strong> even<br />
liquid volumes in vessels.<br />
Pipetting tools<br />
High-precision pipetting tools based on the<br />
<strong>Eppendorf</strong> air cushion liquid h<strong>and</strong>ling<br />
technology. Unique free-jet dispensing<br />
minimizes the risk of cross-contamination.<br />
Manually interchangeable between single<strong>and</strong><br />
eight-channel tools.<br />
Small footprint<br />
Only 65 cm × 48 cm. Fits on even<br />
the smallest available lab bench.<br />
For more details, see also: technical specifications, pages 24 <strong>and</strong> 2 .<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
13<br />
Automated Pipetting | Instruments
Instruments | Automated Pipetting<br />
14<br />
Automated pipetting<br />
epMotion 5075 LH Automated Pipetting System<br />
The epMotion 5075 LH is the ideal solution for advanced liquid<br />
h<strong>and</strong>ling dem<strong>and</strong>s. It offers the same outst<strong>and</strong>ing accuracy <strong>and</strong><br />
precision as the epMotion 5070, making it an excellent tool for<br />
dem<strong>and</strong>ing, small-volume applications such as real-<strong>time</strong> <strong>PCR</strong><br />
set-up or magnetic bead purification, as well as any routine<br />
pipetting task.<br />
Gripper<br />
Move plates to different positions<br />
on the deck.<br />
12-position worktable<br />
Increased batch sizes.<br />
Stacking options for micro <strong>and</strong> deepwell plates.<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
More simplicity—automate your projects faster<br />
than you’ve ever imagined.<br />
“We came to the conclusion that we could automate<br />
complex pipetting steps more quickly <strong>and</strong> simply with<br />
<strong>Eppendorf</strong>’s epMotion liquid h<strong>and</strong>ling platform than<br />
with any other system.”<br />
Neil Campbell, Sr. Experimental Officer,<br />
CMD, Liverpool, UK
epMotion 5075 LH Automated Pipetting System<br />
Automated pipetting with maximum flexibility<br />
The 12 positions, automatic tool exchange, <strong>and</strong> transporting<br />
capabilities exp<strong>and</strong> the application range to h<strong>and</strong>le complex<br />
patterns <strong>and</strong> higher sample numbers. With heating <strong>and</strong> cooling, as<br />
well as the gripper option, the epMotion 5075 LH is one of the most<br />
flexible <strong>and</strong> advanced automated pipetting systems available. Two<br />
options are available to control automated pipetting: epMotion PC<br />
version or epMotion control panel (see page 20).<br />
Automated pipetting<br />
Learn more about the accuracy <strong>and</strong> precision of the epMotion<br />
automated pipetting system. See the applications note on pages<br />
<strong>and</strong> .<br />
Automated tool exchange<br />
Enables the execution of more<br />
complex pipetting patterns.<br />
Plate stacking<br />
Up to five microplates or two deepwell plates<br />
can be stacked on each other by the gripper.<br />
3 thermal element options<br />
Peltier-based thermal element for<br />
heating or cooling of samples or reagents.<br />
For more details, see also: technical specifications, pages 24 <strong>and</strong> 2 .<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
1<br />
Automated Pipetting | Instruments
Instruments | Automated Pipetting<br />
1<br />
Automated pipetting<br />
epMotion 5075 VAC Automated Pipetting System<br />
The epMotion 5075 VAC is all about productivity. With epMotion<br />
5075 VAC’s unique Plug’n’Prep ® applications, you can choose<br />
virtually any kit for your purification needs <strong>and</strong> you are free from the<br />
bench with true walk-away automation. Plug’n’Prep ® also means<br />
it’s easy to set-up—you can start running your protocols<br />
on the day of installation! Two options are available to control<br />
automated pipetting: epMotion PC version or epMotion control<br />
panel (see page 20).<br />
Gripper<br />
Moves plates <strong>and</strong> assembles/disassembles<br />
the vacuum station without manual interference.<br />
11-position worktable<br />
Designed to automate vacuum-based extraction <strong>and</strong><br />
downstream applications for up to 96 samples.<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
More productivity—use your <strong>time</strong><br />
for more important things than tedious<br />
<strong>and</strong> repetitive pipetting tasks.<br />
“The in-house team of automation experts at Promega<br />
has extensively tested <strong>Eppendorf</strong>’s epMotion system<br />
<strong>and</strong> found it to be very simple to operate. End users<br />
were trained in less than a day <strong>and</strong> they were<br />
developing their own protocols almost immediately.”<br />
Dan Kephart, Ph.D. ,<br />
Scientific Application Manager,<br />
Promega Corporation, Madison, Wisconsin, USA
epMotion 5075 VAC Automated Pipetting System<br />
Plug’n’Prep ® vacuum-based separation technology<br />
epMotion 5075 VAC has an 11-position deck plus one vacuum<br />
station for filter plates. The vacuum station is self-adjusting; you<br />
no longer have to “stack collars” of varying sizes around the lower<br />
plate. In addition to the range of pre-tested Plug’n’Prep ® protocols,<br />
alternative purification methods or downstream applications can<br />
be composed individually.<br />
Automated pipetting<br />
Learn more about epMotion 0 VAC nucleic acid purification<br />
applications. See pages 49, 2, 8, <strong>and</strong> 0.<br />
Integrated vacuum pump<br />
No tubing, wiring, <strong>and</strong> reservoirs to maintain.<br />
Silent operation.<br />
Vacuum station<br />
Fully integrated.<br />
Adapts automatically to any filter plate type.<br />
3 thermal element options<br />
Peltier-based thermal element for<br />
heating or cooling of samples or reagents.<br />
For more details, see also: technical specifications, pages 24 <strong>and</strong> 2 .<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
1<br />
Automated Pipetting | Instruments
Instruments | Automated Pipetting<br />
18<br />
Automated pipetting<br />
epMotion 5075 MC Automated Pipetting System<br />
H<strong>and</strong>s-free <strong>PCR</strong><br />
This epMotion version can be equipped with our Mastercycler ® ep<br />
<strong>PCR</strong> system. The integrated cycler enables h<strong>and</strong>s-free set-up<br />
<strong>and</strong> amplification from start to finish without manual intervention.<br />
Automated pipetting with epMotion provides you with more<br />
accuracy <strong>and</strong> performance throughout your day. epMotion frees<br />
you from routine, repetitive pipetting such as setting up <strong>PCR</strong><br />
reactions which gives you more <strong>time</strong> for other tasks in the lab.<br />
Gripper<br />
Moves the plate<br />
to the Mastercycler ® ep.<br />
Automated sealing<br />
Sealing of plates with<br />
reuseable silicon-based<br />
cycle lock system.<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
System features<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
‡ Integrated Mastercycler ® ep software<br />
‡ Fits Mastercycler ® ep gradient with motorized lid<br />
‡ Robust, high-quality aluminum block in 96/384-well format<br />
‡ 96-well silver block for maximum speed<br />
‡ Gripper for plate transport<br />
‡ Multitasking: simultaneous <strong>PCR</strong> reaction <strong>and</strong> liquid h<strong>and</strong>ling<br />
‡ Automatic plate sealing before <strong>PCR</strong><br />
‡ Two options are available to control automated pipetting:<br />
epMotion PC version or epMotion control panel (see page 20).<br />
Interested in real-<strong>time</strong> <strong>PCR</strong>? Read our application example:<br />
Automated real-<strong>time</strong> <strong>PCR</strong> set-up, page 10.<br />
Integrated Mastercycler ® ep<br />
Automated loading<br />
of the cycler.
epMotion 5070 CB Automated Pipetting System<br />
Automated cell culture liquid h<strong>and</strong>ling<br />
epMotion 5070 CB is the first automated pipetting system for<br />
cell culture applications. Now all the benefits of automated<br />
pipetting are available for liquid h<strong>and</strong>ling tasks inside a tissue<br />
culture hood or fume hood.<br />
H<strong>and</strong>ling cells is one of the most tedious tasks in life science<br />
research. epMotion 5070 CB allows you to automate key processes<br />
such as seeding cells, media change, or cytotoxicity tests. It<br />
enables you to h<strong>and</strong>le 6-, 24- or 48-well plates or even denser<br />
formats from 96 to 384 wells, allowing you the best statistical<br />
optimization by using a higher number of samples.<br />
More power—perform applications that you<br />
are not able to h<strong>and</strong>le manually.<br />
“The epMotion is a perfect tool to automate cell culture<br />
liquid h<strong>and</strong>ling. With more reproducible pipetting we were<br />
able to increase the number of data points <strong>and</strong> we<br />
obtained a far better statistical background.”<br />
Dr. René Thierbach,<br />
Institut für Ernährungswissenschaft,<br />
Universität Potsdam, Germany<br />
System features<br />
Automated pipetting<br />
‡ Most commonly used cell culture labware is predefined<br />
for immediate use<br />
‡ Compatible with almost all culture hood br<strong>and</strong>s<br />
‡ Sterile pipetting tips available<br />
‡ Autoclavable pipetting tools<br />
‡ Innovative light barrier detects hood closing<br />
‡ Two options are available to control automated pipetting:<br />
epMotion PC version or epMotion control panel (see page 20).<br />
Compact design<br />
Only 65 cm × 48 cm × 63 cm,<br />
(W × D × H) fits most<br />
bench types.<br />
Light barrier beam<br />
Monitors the closure<br />
of the bench hood.<br />
For more details, see also: technical specifications, pages 24 <strong>and</strong> 2 .<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
19<br />
Automated Pipetting | Instruments
Instruments | Automated Pipetting<br />
20<br />
Automated pipetting<br />
epMotion Control: two smart options<br />
epMotion offers you two options to control automated pipetting:<br />
the epMotion PC version or the epMotion control panel.<br />
Whatever you choose, the innovative epMotion functions make<br />
programming easy; pipetting pattern recognition, labware database,<br />
<strong>and</strong> liquid classes are always included.<br />
epMotion PC version<br />
‡ The network interface of the integrated<br />
PC allows the remote control of the<br />
epMotion via direct ethernet connection.<br />
Method files can be shared using<br />
the computer network or USB port.<br />
epMotion control panel<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
‡ The epMotion PC version with epBlue software offers the most<br />
convenient way ever to use an automated liquid h<strong>and</strong>ling system.<br />
‡ The epMotion control panel provides all functions <strong>and</strong> Windows ®<br />
style programming in an ultra-compact design.<br />
Control panel features<br />
‡ Graphical user interface<br />
‡ Drag <strong>and</strong> drop programming<br />
‡ Windows ® explorer style organization<br />
‡ User management<br />
‡ Run reports<br />
‡ Software preinstalled on the<br />
control panel<br />
Windows is a registered trademark of the Microsoft Corporation<br />
in the United States <strong>and</strong> other countries.
epMotion PC software: epBlue—intuitive operation <strong>and</strong> programming<br />
Simple <strong>and</strong> clear, epBlue guides you through your everyday<br />
pipetting tasks: Choose your objective in the home section<br />
<strong>and</strong> follow the epBlue comm<strong>and</strong>s from left to right with unique<br />
guiding menus <strong>and</strong> the consistent, tab-based structure. With the<br />
unmatched ease-of-use of epBlue, even complex methods can<br />
be generated in minutes.<br />
‡ The home screen provides fast access to the main<br />
functions <strong>and</strong> personal list of the recently used methods,<br />
while the intuitive graphical user interface provides<br />
epMotion editor features<br />
Automated pipetting<br />
Pipetting pattern recognition, labware database, <strong>and</strong> liquid classes<br />
are built-in <strong>and</strong> organized for intuitive use. Simply set up your<br />
worktable, select the pipetting comm<strong>and</strong>s, simulate, <strong>and</strong> launch.<br />
Operating <strong>and</strong> programming epBlue is fast <strong>and</strong> easy.<br />
a consistent, tab-based workflow from left to right. ‡ Menus guide you step by step through the software functions.<br />
epMotion editor—easy off-line program management<br />
epMotion editor<br />
A separate, off-line software package for generating <strong>and</strong> editing<br />
methods as well as printing <strong>and</strong> archiving program processes on<br />
your PC is also available.<br />
‡ Programming functions analogous to the control panel<br />
‡ Graphical user interface identical to epBlue<br />
‡ Print function<br />
‡ Archiving function<br />
‡ Safe operation through 3D run-simulation.<br />
‡ Transfer of methods using the MMC card<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
21<br />
Automated Pipetting | Software
Accesssories | Automated Pipetting<br />
22<br />
Automated pipetting<br />
epMotion accessories<br />
Automate all your favorite labware<br />
Whether 0.2 ml <strong>PCR</strong> tubes, st<strong>and</strong>ard test tubes, 15 or 50 ml blue-cap tubes, six-well plates, 96/384-well microplates, <strong>PCR</strong> plates,<br />
or deepwell plates—with epMotion accessories, you can h<strong>and</strong>le it.<br />
epMotion Accessories<br />
Reservoir rack<br />
Enables provisioning of up to 7<br />
reagent rack modules or reservoirs<br />
with 30 ml or 100 ml filling volume.<br />
Reservoir rack modules TC<br />
Modules for the reservoir rack.<br />
Seven module sizes are available to<br />
use tubes from 0.2 ml <strong>PCR</strong> to<br />
50 ml tubes. Temperature-controlled<br />
when used with a thermal module.<br />
30 ml <strong>and</strong> 100 ml reagent reservoir<br />
The reagent reservoirs are both<br />
“<strong>PCR</strong> clean,” autoclavable, <strong>and</strong><br />
can be placed in the reservoir rack;<br />
their special geometry minimizes<br />
residual volume.<br />
Racks for single test tubes<br />
For micro test tubes,<br />
glass, or plastic tubes.<br />
Thermoadapter DWP 9<br />
For heating or cooling of<br />
deepwell plates. Plates can be<br />
exchanged by the gripper.<br />
Thermoracks for 24 ×<br />
Safelock 0. /1. /2 ml tubes<br />
For 24 micro test tubes.<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
epMotion Accessories<br />
Thermoracks <strong>and</strong> adapter<br />
For the use of 96- or 384-well<br />
<strong>PCR</strong> plates.<br />
Height adapter<br />
Various height adapters enable<br />
exact height level adjustment<br />
<strong>and</strong> accelerated processing of<br />
microplates.<br />
Thermoblock for <strong>PCR</strong> plates<br />
Thermal block holds 96- or<br />
384-well <strong>PCR</strong> plates.<br />
Allows for temperature control<br />
when used with a thermal module.<br />
Gripper for epMotion 0<br />
For the transport of plates<br />
on the worktable <strong>and</strong> for automatic<br />
operation of the vacuum manifold.<br />
Thermal module<br />
(only epMotion 0 )<br />
For racks <strong>and</strong> 96- <strong>and</strong> 384-well<br />
<strong>PCR</strong> plates. Cool or heat up to<br />
three positions on the deck.<br />
More information on epMotion accessories:<br />
order information, page 2 .
epMotion tools <strong>and</strong> tips<br />
Accuracy <strong>and</strong> reproducibility delivered<br />
Based on <strong>Eppendorf</strong> classic air cushion pipetting technology<br />
‡ No tubes, no wires, no air bubbles<br />
‡ As simple to operate <strong>and</strong> maintain as a manual pipette<br />
‡ Excellent pipetting precision, typically below 2% CV at 1 µl*<br />
‡ Automatic alert when pipetting tools need calibration<br />
‡ Ultra-low dead volume pipetting<br />
‡ Every tool tested <strong>and</strong> certified to ISO 85540<br />
* <strong>Eppendorf</strong> application note 168: “Measuring the Accuracy<br />
<strong>and</strong> Precision of the epMotion ® 5070 Workstation<br />
using the Artel Multichannel Verification System (MVS ® ).”<br />
‡ 50 µl filter tip<br />
‡ 50 µl tip without filter<br />
‡ 300 µl filter tip<br />
‡ 300 µl tip without filter<br />
‡ 1,000 µl filter tip<br />
‡ 1,000 µl tip without filter<br />
epT.I.P.S. Motion<br />
Specially selected tips for use in automated systems. Each tip<br />
is inspected for straightness prior to packaging. This ensures a<br />
perfect h<strong>and</strong>ling of 96- <strong>and</strong> 384-well micro <strong>and</strong> <strong>PCR</strong> plates.<br />
‡ Box packaging ensures contamination-free tip loading<br />
‡ Non-carbonized, pure virgin polypropylene, both tip <strong>and</strong> box<br />
are recyclable<br />
‡ St<strong>and</strong>ard, filter, <strong>and</strong> sterile tips available<br />
‡ <strong>PCR</strong>-clean quality grade for filter tips<br />
More information on epMotion tools <strong>and</strong> tips:<br />
order information, pages 24 <strong>and</strong> 2 .<br />
Automated pipetting<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
23<br />
Automated Pipetting | Consumables
Instruments | Automated Pipetting<br />
24<br />
Automated pipetting<br />
epMotion Automated Pipetting Systems<br />
Technical specifications<br />
epMotion 0 LH 0 VAC 0 MC 0 0/ 0 0 CB<br />
Labspace<br />
System Width 140 cm / 56 98 cm / 39<br />
Depth 75 cm / 30 62 cm / 25<br />
Dimensions<br />
Device Width 107 cm / 43 65 cm / 26<br />
Depth 61 cm / 25 48 cm / 20<br />
Height 67 cm / 27 63 cm / 26<br />
Control panel Width 25 cm / 10<br />
Depth 15 cm / 6<br />
Height 11 cm / 4.4<br />
Weight<br />
Device 85 kg / 188 lb 90 kg / 199 lb 102 kg / 225 lb 45 kg / 99 lb<br />
Control panel 1.2 kg / 3 lb<br />
Power supply<br />
Voltage 100 to 130 V ±10% / 200 to 240 V ±10%<br />
Frequency 50 to 60 Hz ±5%<br />
Max. output 1,000 W 70 W<br />
Ambient conditions<br />
Air temperature storage –20 to +70 °C / –4 to +158 °F<br />
operation +15 to +35 °C / +59 to +95 °F<br />
Atmospheric humidity storage 10 to 80% rF / rH<br />
operation 55 to 75% rF / rH<br />
Atmospheric pressure storage 300 to 1,060 hPa / 4.4 to 15.4 psi<br />
operation 970 to 1,060 hPa / 14.1 to 15.4 psi<br />
Pipetting performance specification according to ISO 8<br />
1-channel tool Volume systematic measurement error r<strong>and</strong>om measurement error<br />
TS 0 1 µl ±15 % ≤5 %<br />
50 µl ±1.2 % ≤0.4 %<br />
TS 300 20 µl ±4 % ≤2.5 %<br />
300 µl ±0.6 % ≤0.3 %<br />
TS 1000 40 µl ±5 % ≤1.5 %<br />
1,000 µl ±0.7 % ≤0.15 %<br />
Typical pipetting performance*<br />
MVS data for epMotion 0 0<br />
single-channel dispensing tools<br />
Dispensing<br />
tool<br />
Target vol.<br />
(μl)<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
Coefficient<br />
of variation (%)<br />
TS 0 1 1.9<br />
50 0.2<br />
TS 300 30 0.4<br />
200 0.3<br />
TS 1000 40 0.6<br />
200 0.3<br />
* <strong>Eppendorf</strong> application note 168: “Measuring the Accuracy <strong>and</strong> Precision of the epMotion ® 5070<br />
Workstation Using the Artel Multichannel Verification System (MVS ® ).”<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
in pipetting mode, free jet, without pre-wetting, with distilled water, at 20 °C<br />
MVS data for the epMotion 0 0<br />
multichannel dispensing tools<br />
Dispensing<br />
tool<br />
Target vol.<br />
(μl)<br />
Coefficient<br />
of variation (%)<br />
TM 0-8 1 1.6<br />
50 0.4<br />
TM 300-8 20 1.9<br />
200 0.4<br />
TM 1000-8 45 0.8<br />
200 0.3
epMotion Automated Pipetting Systems<br />
Technical specifications<br />
Automated pipetting<br />
epMotion 0 LH 0 VAC 0 MC 0 0/ 0 0 CB<br />
Conductor<br />
X, Y, Z axes systematic measurement error r<strong>and</strong>om measurement error<br />
Positioning ±0.3 mm ±0.1 mm<br />
Positions in MTP format 12 12 10 4<br />
Detector<br />
Optical confocal infrared detector contact-free detection of liquid levels, tools used,<br />
labware worktables, tip types, <strong>and</strong> quantities<br />
Optical Sensor liquid surface must be 90 ±3° to the vertical plane of the optical sensor<br />
Gripper<br />
Carrying capacity ≤1,200 g<br />
Vacuum unit<br />
Max. output - (feed) 35 l/min - -<br />
Suction range - 0.1 to 0.85<br />
±0.05 hPa<br />
- -<br />
Suction <strong>time</strong> - 1 to 99 minutes - -<br />
Tempering unit: optional<br />
Setting range 0 °C to 110 °C -<br />
Heating <strong>time</strong> of the heating-cooling plate from 25 °C to 95 °C in 8 minutes -<br />
Cooling <strong>time</strong> of the heating-cooling plate from 25 °C to 4 °C in 5 minutes -<br />
Software<br />
Operating Software MotionManager v. 4.0 or higher; Firmware: MotionInstrument v. 4.0 or higher;<br />
Labware 1.0 or higher<br />
Ordering information<br />
Description Catalog No.<br />
epMotion 0 0<br />
epMotion 0 0 with control panel<br />
Basic device incl. control panel, mouse, software, waste box,<br />
MMC, <strong>and</strong> reader, 50/60 Hz, 100–240 V.<br />
epMotion 0 0 PC version<br />
Same as 5070 catalog no. 960000005 but with integrated industrial PC, keyboard, <strong>and</strong> mouse<br />
instead of control panel. Software <strong>and</strong> monitor not included.<br />
epMotion 0 0 CB with control panel<br />
epMotion to be operated inside closed containers, basic device<br />
incl. control panel, mouse, software, waste box, MMC, <strong>and</strong> reader 100–240 V.<br />
epMotion 0 0 CB with integrated PC<br />
Same as 5070 catalog no. 960000021 but with integrated industrial PC, keyboard, <strong>and</strong> mouse<br />
instead of control panel. Software <strong>and</strong> monitor not included.<br />
epMotion 0<br />
epMotion 0 LH<br />
Basic device incl. control panel, software, waste box,<br />
MMC, <strong>and</strong> reader, 50/60 Hz, 100–240 V.<br />
epMotion 0 VAC<br />
Basic device incl. control panel, software, vacuum system,<br />
gripper, waste box, MMC, <strong>and</strong> reader, 50/60 Hz, 100–240 V.<br />
epMotion 0 MC<br />
Basic device incl. control panel, software, cycler bay, gripper,<br />
waste box, MMC, <strong>and</strong> reader, 50/60 Hz, 100–240 V. Mastercycler ® not included.<br />
epMotion 0 LH PC version<br />
Same as 5075 catalog no. 960020006 but with integrated industrial PC, keyboard, <strong>and</strong> mouse<br />
instead of control panel. Software <strong>and</strong> monitor not included.<br />
epMotion 0 VAC PC version<br />
Same as 5075 catalog no. 960020014 but with integrated industrial PC, keyboard, <strong>and</strong> mouse<br />
instead of control panel. Software <strong>and</strong> monitor not included.<br />
epMotion 0 MC PC version<br />
Same as 5075 catalog no. 960020022 but with integrated industrial PC, keyboard, <strong>and</strong> mouse<br />
instead of control panel. Software <strong>and</strong> monitor not included.<br />
960000005<br />
960000100<br />
960000021<br />
960000200<br />
960020006<br />
960020014<br />
960020022<br />
960020101<br />
960020202<br />
960020303<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
2<br />
Automated Pipetting | Instruments
Accessories | Automated Pipetting<br />
2<br />
Automated pipetting<br />
epMotion Automated Pipetting Systems<br />
Ordering information<br />
Description Catalog No.<br />
Accessories epMotion PC versions<br />
Monitor<br />
19 TFT monitor to be used with epMotion versions with integrated PC.<br />
epBlue – epMotion PC software<br />
Preinstalled operating software for epMotion versions with integrated PC,<br />
epBlue must be ordered with each epMotion PC version.<br />
Upgrade <strong>and</strong> conversion kits<br />
Upgrade Set 1<br />
Upgrade kit for epMotion 5075<br />
with serial numbers 1,000 (integrated PC)<br />
Conversion kit MC<br />
For conversion of LH version in MC version<br />
(Mastercycler ® not included)<br />
Conversion kit VAC<br />
For conversion of LH version in VAC version<br />
(vacuum system included)<br />
Dispensing tools <strong>and</strong> gripper<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
960021044<br />
960000309<br />
960021022<br />
960021033<br />
960021002<br />
960021011<br />
Holder for up to six pipetting tools 960001109<br />
Dispensing tool TS 0<br />
960001010<br />
1-channel dispensing tool for the volume range from 1–50 µl<br />
Dispensing tool TS 300<br />
960001028<br />
1-channel dispensing tool for the volume range from 20–300 µl<br />
Dispensing tool TS 1000<br />
960001036<br />
1-channel dispensing tool for the volume range from 40–1,000 µl<br />
Dispensing tool TM 0-8<br />
960001044<br />
8-channel dispensing tool for the volume range from 1–50 µl<br />
Dispensing tool TM 300-8<br />
960001052<br />
8-channel dispensing tool for the volume range from 20–300 µl<br />
Dispensing tool TM 1000-8<br />
960001061<br />
8-channel dispensing tool for the volume range from 40–1,000 µl<br />
epT.I.P.S. Motion<br />
epT.I.P.S. Motion 0 μl, volume range 1–50 µl,<br />
15 × 96 tips in racks, <strong>Eppendorf</strong> quality<br />
epT.I.P.S. Motion 0 μl, filter, volume range 1–50 µl,<br />
15 × 96 tips in racks, <strong>PCR</strong>-clean<br />
epT.I.P.S. Motion 0 μl, sterile, volume range 1–50 µl,<br />
15 × 96 tips in racks<br />
epT.I.P.S. Motion 0 μl, sterile, filter, volume range 1–50 µl,<br />
15 × 96 tips in racks, <strong>PCR</strong>-clean<br />
epT.I.P.S. Motion 300 μl, volume range 20–300 µl,<br />
15 × 96 tips in racks, <strong>Eppendorf</strong> quality<br />
epT.I.P.S. Motion 300 μl, filter, volume range 20–300 µl,<br />
15 × 96 tips in racks, <strong>PCR</strong>-clean<br />
epT.I.P.S. Motion 300 μl, sterile, volume range 20–300 µl,<br />
15 × 96 tips in racks<br />
epT.I.P.S. Motion 300 μl, sterile, filter, volume range 20–300 µl,<br />
15 × 96 tips in racks, <strong>PCR</strong>-clean<br />
epT.I.P.S. Motion 1,000 μl, volume range 40–1,000 µl,<br />
15 × 96 tips in racks, <strong>Eppendorf</strong> quality<br />
epT.I.P.S. Motion 1,000 μl, filter, volume range 40–1,000 µl,<br />
15 × 96 tips in racks, <strong>PCR</strong>-clean<br />
epT.I.P.S. Motion 1,000 μl, sterile, volume range 40–1,000 µl,<br />
15 × 96 tips in racks<br />
epT.I.P.S. Motion 1,000 μl, sterile, filter, volume range 40–1,000 µl,<br />
15 × 96 tips in racks, <strong>PCR</strong>-clean<br />
960050002<br />
960050029<br />
960050200<br />
960050201<br />
960050045<br />
960050061<br />
960050202<br />
960050203<br />
960050088<br />
960050100<br />
960050204<br />
960050205
epMotion Automated Pipetting Systems<br />
Ordering information<br />
Automated pipetting<br />
Description Catalog No.<br />
Reagent rack, reservoirs, modules<br />
Reservoir Rack for use with 30 ml <strong>and</strong> 100 ml reagent reservoirs<br />
<strong>and</strong> reagent rack modules.<br />
Reservoir Rack Module TC <strong>PCR</strong> 0.2 ml<br />
Temperature-controlled for heating or cooling of 8 × 0.2 ml <strong>PCR</strong> tubes,<br />
to be used with a reservoir rack.<br />
Reservoir Rack Module TC <strong>PCR</strong> 0. ml<br />
Temperature-controlled for heating or cooling of 8 × 0.5 ml <strong>PCR</strong> tubes,<br />
to be used with a reservoir rack.<br />
Reservoir Rack Module TC Safe Lock Tubes<br />
Temperature-controlled for heating or cooling of 4 × 0.5/1.5/2.0 ml tubes,<br />
to be used with a reservoir rack.<br />
Reservoir Rack Module TC Ø 12 mm<br />
Temperature-controlled for heating or cooling of 4 × 12 mm tubes,<br />
to be used with a reservoir rack.<br />
Reservoir Rack Module TC Ø 1 mm<br />
Temperature-controlled for heating or cooling of 4 × 16 mm tubes,<br />
must be used with a reservoir rack.<br />
Reservoir Rack Module TC Ø 1 ml “BlueCap”<br />
Temperature-controlled for heating or cooling of 4 × 17 mm tubes,<br />
to be used with a reservoir rack, needs two module positions.<br />
Reservoir Rack Module TC Ø 0 ml “BlueCap”<br />
Temperature-controlled for heating or cooling of 2 × 29 mm tubes,<br />
to be used with a reservoir rack, needs two module positions.<br />
epMotion Reservoir 30 ml<br />
Must be used with a Reservoir Rack. 30 ml maximum volume,<br />
dead volume optimized. 5 reservoirs packed in separate bags, 10 bags per set.<br />
All reservoirs are <strong>PCR</strong>-clean (free of human DNA, DNase, RNase, <strong>and</strong> <strong>PCR</strong> inhibitors)<br />
<strong>and</strong> made of polypropylene. Production batch tested <strong>and</strong> certified.<br />
epMotion Reservoir 100ml<br />
Same as catalog no. 960051009, but with 100 ml max volume.<br />
Reservoir Rack Module Adapter TC 30 ml<br />
Together with a thermal module, enables the temperature control<br />
of 1 × epMotion reservoir 30 ml, must be used with a reservoir rack<br />
<strong>and</strong> a tube reservoir rack module.<br />
Reservoir Rack Module Adapter TC 100 ml<br />
Same as catalog no. 960002670, but for 100 ml reservoirs.<br />
Racks for single tubes, no temperature control, for a supply of <strong>Eppendorf</strong> tubes ® , glass or plastic test tubes.<br />
960002148<br />
960002601<br />
960002611<br />
960002620<br />
960002630<br />
960002640<br />
960002650<br />
960002660<br />
960051009<br />
960051017<br />
960002670<br />
960002680<br />
Rack for 24× test tubes, Ø 17 mm × 100 mm max. length 960002024<br />
Rack for 24× test tubes, Ø 17 mm × 60 mm max. length 960002156<br />
Rack for 24× test tubes, Ø 16 mm × 100 mm max. length 960002032<br />
Rack for 24× test tubes, Ø 16 mm × 60 mm max. length 960002164<br />
Rack for 24× test tubes, Ø 13 mm × 100 mm max. length 960002041<br />
Rack for 24× test tubes, Ø 12 mm × 100 mm max. length 960002059<br />
Rack for 24× test tubes, Ø 14 mm × 100 mm max. length 960002369<br />
Rack for 24× test tubes, Ø 15 × 100 mm max. length 960002377<br />
Rack for 24× test tubes, Ø 15 × 60 mm max. length 960002326<br />
Rack for 24× test tubes, Ø 14 × 60 mm max. length 960002334<br />
Rack for 24× test tubes, Ø 13 × 60 mm max. length 960002342<br />
Rack for 24× test tubes, Ø 12 × 60 mm max. length 960002351<br />
Rack for 9 × 1. /2.0 ml screw-cap tubes,<br />
Two slots of the worktable are blocked, only pipetting mode compatible.<br />
960002318<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
2<br />
Automated Pipetting | Accessories
Accessories | Automated Pipetting<br />
28<br />
Automated pipetting<br />
epMotion Automated Pipetting Systems<br />
Ordering information<br />
Description Catalog No.<br />
Height adapters for uniform levels of labware, this enables faster processing of the plate.<br />
Height adapter, 85 mm 960002105<br />
Height adapter, 55 mm 960002113<br />
Height adapter, 40 mm for tips 960002121<br />
Thermoblocks, Thermoracks, <strong>and</strong> Thermoadapter<br />
Thermoadapter Frosty For cooling of skirted <strong>PCR</strong> plates by integration<br />
of <strong>PCR</strong> Cooler into epMotion adapter system.<br />
960002300<br />
Thermorack for 24× Safe-Lock 0.5 ml tubes, temperature control. 960002067<br />
Thermorack for 24× Safe-Lock 1.5 / 2 ml tubes, 1.5 / 2 ml tubes,<br />
temperature control. For a supply of 24× 1.5 ml or 2.0 ml test tubes.<br />
960002075<br />
Adapter 25× Safe Lock 0.5 ml tubes. 960002172<br />
Thermoadapter for 9 -<strong>PCR</strong> Skirted, for heating or cooling of <strong>PCR</strong> plates,<br />
plates are exchangeable via the gripper.<br />
960002199<br />
Thermoadapter for 384-<strong>PCR</strong> Skirted, for heating or cooling of <strong>PCR</strong> plates,<br />
plates are exchangeable via the gripper.<br />
960002202<br />
Thermoblock for <strong>PCR</strong> 96 wells / tubes 0.2 ml. For use of 48 × 0.2 ml tubes. 960002083<br />
Thermoblock for <strong>PCR</strong> 384 wells, for use of a <strong>PCR</strong> 384-well plate. 960002091<br />
Thermoadapter DWP 9<br />
For use with 1.1 ml <strong>Eppendorf</strong> deepwell plates.<br />
960002391<br />
Additional accessories<br />
epMotion Editor, complete version Incl. editor key, software package for creating <strong>and</strong> editing methods,<br />
960000269<br />
<strong>and</strong> for printing out archiving program sequences on a PC.<br />
epMotion Editor, additional license<br />
960000300<br />
License for a second PC, USB key.<br />
Must be used with an epMotion editor package 5075.014.009.<br />
MultiMediaCard, empty for archiving parameters<br />
960002008<br />
<strong>and</strong> transport of data between control panel <strong>and</strong> PC.<br />
Wastebox Receptacle for used pipette tips. 960002016<br />
Assembly plate To be used with epMotion 5070 CB,<br />
960002570<br />
supports laminar flow worktable.<br />
Additional accessories only for epMotion 0<br />
Thermal module<br />
960002181<br />
Heats or cools microplates or other labware on the epMotion 5075.<br />
Gripper<br />
960002270<br />
Moves plates <strong>and</strong> vacuum station components on the epMotion 5075.<br />
Gripper holder<br />
960002211<br />
St<strong>and</strong> for the gripper on the worktable<br />
Vac Holder Base for the Vac Frame 960002237<br />
Vac Lid + Mats Lid for the vacuum manifold 960002245<br />
Mats for Vac Lid 960002407<br />
Vac Frame 1 to adapt different filter plates to the vacuum manifold. 960002253<br />
Vac Frame 2 to adapt different filter plates to the vacuum manifold. 960002261<br />
CycleLock starter set<br />
960002288<br />
1 frame <strong>and</strong> 8 mats for automated sealing of <strong>Eppendorf</strong> <strong>PCR</strong> plates.<br />
Can only be used with Mastercyler ep <strong>and</strong> motorized lid.<br />
All mats are <strong>PCR</strong>-clean (free of human DNA, DNase, RNase, <strong>and</strong> <strong>PCR</strong> inhibitors).<br />
Production batch tested <strong>and</strong> certified.<br />
CycleLock Mats<br />
960002296<br />
5 sealing mats, frame not included. All mats are <strong>PCR</strong>-clean.<br />
Production batch tested <strong>and</strong> certified.<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong><br />
ARTS<br />
29
Instruments | <strong>Real</strong>-<strong>time</strong> <strong>PCR</strong><br />
30<br />
Thermal Cycler<br />
Mastercycler® ep realplex<br />
Application<br />
‡ Quantitative real-<strong>time</strong> <strong>PCR</strong><br />
Product features<br />
‡ Quick detection<br />
‡ Choose from fast or<br />
ultra-high-speed models<br />
‡ Unrestricted system gives<br />
you total freedom to use the<br />
tubes, plates <strong>and</strong> reagents of<br />
your choice<br />
‡ Modular design provides<br />
several system options<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
‡ Compact footprint saves<br />
valuable bench space<br />
‡ Solid design for quiet<br />
operation <strong>and</strong> dependability<br />
‡ Intuitive software includes a<br />
variety of analysis modules for<br />
easy translation of results<br />
Quality in all forms<br />
The new optical system of the Mastercycler ep realplex offers a<br />
new proprietary particle protection technology* <strong>and</strong> very sensitive<br />
photomultipliers of the latest generation.<br />
Small, fast <strong>and</strong> flexible<br />
Mastercycler ep realplex meets all the latest requirements of<br />
quantitative <strong>PCR</strong> applications: high-speed temperature ramping,<br />
short detection <strong>time</strong>s <strong>and</strong> intuitive assay programming translate to<br />
huge <strong>time</strong> savings, so that more experiments can be completed per<br />
day—with accurate <strong>and</strong> reliable results. And its flexibility is beyond<br />
compare—this completely open system allows you to use tubes,<br />
plates <strong>and</strong> reagents of your choice.<br />
Modular <strong>and</strong> compact in size, realplex can fit in virtually any lab,<br />
no matter how limited the bench space. Since it is part of the<br />
premier Mastercycler ep family of thermal cyclers, if you already<br />
have a Mastercycler ep gradient S in your lab, for example, you can<br />
upgrade it to a realplex real-<strong>time</strong> <strong>PCR</strong> cycler at any <strong>time</strong>; <strong>and</strong> the<br />
realplex 2 module can be replaced by the realplex 4 module if/when<br />
greater multiplexing becomes essential.<br />
<strong>Eppendorf</strong> Thermal Sample Protection technology prevents the<br />
negative effects of condensation on amplification, <strong>and</strong> a solid<br />
construction with minimal moving parts makes this a quiet,<br />
dependable cycler that promotes consistently high sensitivity.<br />
*Patent pending.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Practice of the patented polymerase chain reaction (<strong>PCR</strong>) process<br />
requires a license. The <strong>Eppendorf</strong> Thermal Cycler is an Authorized<br />
Thermal Cycler <strong>and</strong> may be used with <strong>PCR</strong> licenses available<br />
from Applied Biosystems. Its use with Authorized Reagents also<br />
provides a limited <strong>PCR</strong> license in accordance with the label rights<br />
accompanying such reagents. This is a Licensed <strong>Real</strong>-Time Thermal<br />
Cycler under Applera’s United States Patent No. 6,814,934 <strong>and</strong><br />
corresponding claims in non-U.S. counterparts thereof, for use<br />
in research <strong>and</strong> for all other applied fields except human in vitro<br />
diagnostics. No right is conveyed expressly, by implication or by<br />
estoppel under any other patent claim.
Mastercycler® ep realplex<br />
realplex thermoblocks<br />
Choose either the 96-well aluminum or the 96-well silver<br />
thermoblock: both feature <strong>Eppendorf</strong> SteadySlope ® gradient<br />
technology for assay optimization—even optimization of two-step<br />
q<strong>PCR</strong>. If you require the fastest heating <strong>and</strong> cooling rates, choose<br />
the silver block, whose performance is enhanced by our unique<br />
Impulse <strong>PCR</strong> technology (more information on page 36).<br />
Both blocks are compatible with either the realplex 2 or realplex 4<br />
optical modules, which permit the use of nearly all fluorescent dyes<br />
used in real-<strong>time</strong> <strong>PCR</strong>: the realplex 2 module has two fluorescence<br />
filters <strong>and</strong> one channel photo-multiplier tube (CPM), while the<br />
realplex 4 module incorporates four filters <strong>and</strong> two CPMs for up<br />
to four-fold multiplexing assays. Our new <strong>and</strong> innovative CPMs,<br />
in contrast to conventional photo-multiplier tubes, are far less<br />
sensitive to magnetic field interference <strong>and</strong> offer greatly<br />
increased sensitivity.<br />
Licensed for real-<strong>time</strong> <strong>PCR</strong>, see previous page.<br />
realplex system product highlights begin on page 34.<br />
96-well excitation<br />
Thermal Cycler<br />
The fluorescent dyes chosen for each experiment are excited by<br />
96 individual LEDs, which have a substantially longer lifespan than<br />
halogen lamps: a blue light emission of ~470 nm excites nearly all<br />
applicable fluorophores, including SYBR ® Green, FAM, VIC, TET,<br />
HEX, ROX, JOE, TAMRA <strong>and</strong> more; the emitted fluorescence is<br />
focused through an array of lenses <strong>and</strong> passed to the optical<br />
detection unit through 96 individual optical fibers, where our new<br />
CPMs serve as the detectors.<br />
Results in real-<strong>time</strong><br />
realplex software offers six different evaluation modules for<br />
maximum flexibility when processing results. An intuitive user<br />
interface ensures quick <strong>and</strong> easy <strong>PCR</strong> <strong>and</strong> assay setup, as well<br />
as simple transfer of previously established <strong>PCR</strong> protocols.<br />
Smart programming automatically interprets possible dye<br />
interferences when performing multiplex assays, <strong>and</strong> results<br />
are easily exported to Microsoft ® Excel for GLP compliance<br />
(as an alternative to the built-in analysis modes).<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
31<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> | Instruments
Instruments | <strong>Real</strong>-<strong>time</strong> <strong>PCR</strong><br />
32<br />
Thermal Cycler<br />
Mastercycler® ep realplex<br />
‡ Plate layout<br />
The clear <strong>and</strong> comprehensive organization of multiple viewing<br />
windows, as well as intuitive options for selection within each<br />
view, enable easy <strong>and</strong> fast plate setup when creating assays.<br />
‡ Quantification/relative quantification<br />
This analysis module performs calculations from raw data<br />
for a variety of assays, including absolute quantification<br />
of DNA or relative quantification of gene expression based<br />
on the DDC t method.<br />
‡ Endpoint<br />
The analysis module option for endpoint measurements instructs<br />
Mastercycler ep realplex to serve as a “plate fluorometer” <strong>and</strong> aids<br />
in the determination of absolute fluorescence intensities, even with<br />
samples previously amplified on st<strong>and</strong>ard thermocyclers.<br />
Licensed for real-<strong>time</strong> <strong>PCR</strong>, see page 30.<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
‡ Raw data<br />
This software module displays the generated raw data in real <strong>time</strong>,<br />
which means that you can immediately evaluate your real-<strong>time</strong> <strong>PCR</strong><br />
<strong>and</strong> interrupt the reaction as soon as the desired result is obtained.<br />
‡ Gene identification<br />
Selecting this analysis module option generates allele-discrimination<br />
results. Data previously generated in melting curve analyses, end-<br />
point measurements or C t-value determinations serves as the basis.<br />
‡ +/– Assay<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Define critical threshold values through this analysis module<br />
option to differentiate between positive <strong>and</strong> negative samples<br />
(e.g., for the detection of pathogens). Data from previous endpoint<br />
measurements serves as the source for such determinations.
Mastercycler® ep realplex<br />
Technical specifications<br />
Optical module<br />
Excitation source: 96 LEDs (470 nm)<br />
Emission filters: 520 nm/550 nm/580 nm/605 nm (realplex 4 )<br />
520 nm/550 nm (realplex 2 )<br />
Detector: 2-channel photo-multiplier tubes (realplex 4 )<br />
1-channel photo-multiplier tube (realplex 2 )<br />
Dynamic range: 9 orders of magnitude from starting copy number<br />
Sensitivity: ≤ 50 fM fluorescein<br />
Thermomodule<br />
Sample capacity: 96 x 0.2 ml <strong>PCR</strong> tubes or one 96 <strong>PCR</strong> plate<br />
(unskirted, semiskirted or skirted—as per SBS st<strong>and</strong>ard)<br />
Temperature control range of block: 4 °C–99 °C<br />
Degree range of gradient, maximum: 1 °C–24 °C (thermomodule Mastercycler ep S)<br />
1 °C–20 °C (thermomodule Mastercycler ep)<br />
Temperature control range of gradient: 30 °C–99 °C<br />
Temperature of lid: 105 °C<br />
Block homogeneity: 35 °C ± 0.3 °C<br />
90 °C ± 0.4 °C<br />
Control accuracy: ± 0.2 °C<br />
Heating speed*: approx. 6 °C/s (thermomodule Mastercycler ep S)<br />
approx. 4 °C/s (thermomodule Mastercycler ep)<br />
Cooling speed*: approx. 4.5 °C/s (thermomodule Mastercycler ep S)<br />
approx. 3 °C/s (thermomodule Mastercycler ep)<br />
Complete system<br />
Dimensions (W x D x H): 10.2 x 16.1 x 15.6 in/26 x 41 x 39.6 cm<br />
Total weight: 53 lb/24 kg<br />
Weight of thermomodule: 37.5 lb/17 kg<br />
Weight of detection module: 15.4 lb/7 kg<br />
Voltage requirements: 100–130 V/50–60 Hz<br />
Power consumption: 800 W<br />
*Measured at block<br />
Ordering information<br />
Thermal Cycler<br />
Description Catalog No.<br />
Mastercycler ® ep realplex:<br />
Mastercycler ® ep realplex 2 , with aluminum block <strong>and</strong> two emission filters Please inquire<br />
Mastercycler ® ep realplex 2 S, with silver block <strong>and</strong> two emission filters<br />
Mastercycler ® ep realplex 4 , with aluminum block <strong>and</strong> four emission filters<br />
Mastercycler ® ep realplex 4 S, with silver block <strong>and</strong> four emission filters<br />
Optical detection module to upgrade Mastercyler ep gradient / S<br />
realplex 2 Please inquire<br />
realplex 4<br />
Service<br />
Performance Plans Please inquire<br />
Validation/calibration service<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
33<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> | Instruments
Instruments | <strong>Real</strong>-<strong>time</strong> <strong>PCR</strong><br />
34<br />
Thermal Cycler<br />
Product highlights—Mastercycler® ep realplex<br />
Gradient function: a highly useful tool for optimizing real-<strong>time</strong> <strong>PCR</strong><br />
The right choice of annealing temperature for primers (<strong>and</strong> probe)<br />
has a significant influence on the performance <strong>and</strong> efficiency of<br />
real-<strong>time</strong> <strong>PCR</strong> reactions. While software programs offer various<br />
algorithms to estimate this temperature, the best way to accurately<br />
determine optimal annealing temperature is empirically.<br />
Mastercycler ep realplex’s gradient function 1 offers a wide<br />
temperature range across all 12 column well positions of the<br />
thermoblock, <strong>and</strong> it provides 12 different temperatures to test in<br />
a single experiment: choose a temperature span from 1 °C up to<br />
20 °C when using the aluminum block, or from 1 °C to 24 °C when<br />
using the silver block.<br />
The gradient function is easy to use, <strong>and</strong> its importance for real-<br />
<strong>time</strong> <strong>PCR</strong> optimization is demonstrated in the following assay.<br />
‡ Fig. 1: Simple programming of realplex’s annealing<br />
temperature gradient step<br />
The gradient preview for the 12 column well positions on the block<br />
demonstrate the highly linear characteristic of its Triple Circuit<br />
Peltier design.<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> with gradient function—assay setup<br />
A mastermix containing <strong>Real</strong>MasterMix 2 , SYBR ® Green I <strong>and</strong> prim-<br />
ers was prepared to amplify <strong>and</strong> detect a sequence segment of<br />
the Beta globin gene. A temperature gradient over a range of 24 °C<br />
(from 44 °C to 68 °C) on the Mastercycler ep realplex S (silver block)<br />
was programmed for the annealing step (Fig. 1). The program in<br />
detail: 95 °C for 2 min; 40 cycles of 95 °C for 15 s, 44 °C–68 °C for<br />
30 s, 68 °C for 60 s; <strong>and</strong> finally a melting curve analysis.<br />
For each of the 12 different annealing temperatures, 3 sample<br />
replicates, each with 5 ng of human genomic DNA as template,<br />
<strong>and</strong> one negative template control (NTC) were prepared.<br />
1 U.S. Pat. 6,767,512.<br />
2 U.S. Pat. 6,667,165.
Product highlights—Mastercycler® ep realplex<br />
Gradient function: a highly useful tool for optimizing real-<strong>time</strong> <strong>PCR</strong>, continued.<br />
Thermal Cycler<br />
Well position 1 2 3 4 5 6 7 8 9 10 11 12<br />
Annealing<br />
temperature (°C):<br />
44.1 44.7 46.2 48.6 51.4 54.6 57.8 61.0 63.8 66.0 67.4 67.8<br />
Mean C t: 28.5 28.7 28.1 26.9 25.6 25.0 24.3 24.1 24.0 23.9 24.1 24.4<br />
Specific <strong>PCR</strong> product: - - (+) + + + + + + + + +<br />
Nonspecific<br />
<strong>PCR</strong> product:<br />
+ + + (+) - - - - - - - -<br />
‡ Table 1: Analysis parameters of the three replicates for each well position<br />
Results <strong>and</strong> conclusions<br />
At temperatures below an annealing temperature of 48.6 °C<br />
(column well positions 1–3) there are primarily nonspecific <strong>PCR</strong><br />
products (Table 1, Fig. 3). Nonspecific products were produced in<br />
some NTCs (no template controls) at these temperatures as well.<br />
Therefore, when a double-str<strong>and</strong>ed DNA-binding dye such as<br />
SYBR ® Green I is used for detection, it is important to check the<br />
products with a melting curve analysis following the last cycle of<br />
the <strong>PCR</strong> reaction (Fig. 3).<br />
The Ct values of the specific Beta globin <strong>PCR</strong> products vary from<br />
approx. 24 to 28 (Table 1, Fig. 2). This means that there is a Ct shift of approx. 4 <strong>PCR</strong> cycles from the most favorable annealing<br />
temperatures (column well positions 7–12) to the least favorable<br />
(column well position 3) annealing temperatures—a very significant<br />
determination that impacts the practice of real-<strong>time</strong> <strong>PCR</strong>. Note<br />
that Table 1 indicates the optimal annealing temperature in column<br />
10—<strong>and</strong> a 0.5 Ct shift over an ~2 °C change in temperature from<br />
column 10 to column 12. Thus, we see that the Ct values drop<br />
(improve) from columns 1 to 10 <strong>and</strong> then start to increase again<br />
from 10 to 12.<br />
This assay demonstrates the importance of gradient optimization<br />
of annealing temperatures to achieve reliable results across<br />
individual assays <strong>and</strong> experiments. The flexible gradient function<br />
of Mastercycler ep realplex is a <strong>time</strong>- <strong>and</strong> cost-saving feature,<br />
especially for introducing <strong>and</strong> establishing new real-<strong>time</strong> <strong>PCR</strong><br />
reactions in the laboratory.<br />
‡ Fig. 2: Logarithmic-scaled amplification plots of the<br />
Beta globin assay<br />
The range of C t values correspond to a range in annealing<br />
temperature in a gradient optimization experiment.<br />
‡ Fig. 3: Peak curves (negative first derivative of the melting<br />
curves) of each of the three replicates from column positions<br />
3 <strong>and</strong> 8<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
35<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> | Instruments
Instruments | <strong>Real</strong>-<strong>time</strong> <strong>PCR</strong><br />
36<br />
realplex highlights<br />
Product highlights—Mastercycler® ep realplex<br />
Impulse <strong>PCR</strong>: a novel, accelerated <strong>and</strong> improved q<strong>PCR</strong> Hot Start method<br />
An essential <strong>and</strong> critical goal during any <strong>PCR</strong> is to avoid nonspecific<br />
primer annealing during <strong>PCR</strong> setup <strong>and</strong> each successive cycle<br />
of <strong>PCR</strong>. Two key <strong>and</strong> unique features of realplex—SteadySlope ®<br />
gradient technology <strong>and</strong> Impulse <strong>PCR</strong>—make this goal easy<br />
to achieve.<br />
As discussed in the “Gradient function: a highly useful tool for<br />
optimizing real-<strong>time</strong> <strong>PCR</strong>” highlight on the previous pages, specific<br />
primer annealing is often highly sensitive for optimal temperature. In<br />
addition, a nonoptimal annealing temperature can have a negative<br />
impact on the C t values <strong>and</strong> the overall sensitivity of the assay. With<br />
the gradient function, optimization of a specific probe <strong>and</strong>/or primer<br />
pair’s ideal annealing temperature is easy <strong>and</strong> fast, <strong>and</strong> an optimized<br />
real-<strong>time</strong> <strong>PCR</strong> cycling program can be created in a short <strong>time</strong>.<br />
With a well-designed assay, once the optimal annealing<br />
temperature has been determined <strong>and</strong> adopted, the risk of<br />
nonspecific amplification after the first cycle is minimal. This is<br />
due to the fact that the temperature for specific annealing will not<br />
Temp.<br />
1<br />
‡ Fig. 1: A typical <strong>PCR</strong> cycling program<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
2<br />
2<br />
Time<br />
The critical temperature range of initial ramping (circled), lower than<br />
the critical annealing temperature (Step 2), is where nonspecific<br />
amplification will most likely occur.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
fall below the critical temperature at which nonspecific annealing<br />
occurs. However, the first initial ramping step of a <strong>PCR</strong> carries a<br />
serious risk of nonspecific amplification—<strong>and</strong>, therefore, lowered<br />
specificity—for two reasons: (a) in the course of ramping from<br />
ambient to initial denaturation temperature, the sample heats across<br />
a range of temperatures—at first well below that of the optimal an-<br />
nealing temperature, <strong>and</strong> (b) any nonspecific product extended<br />
in that first ramping step will have the potential for amplification<br />
in each <strong>and</strong> every additional cycle. This subjects the assay to<br />
additional potential for nonspecific amplification to occur.<br />
To demonstrate the effects of nonspecific amplification: if<br />
one of several suboptimal events occurs—such as choice of<br />
wrong annealing temperature for the cycle programming, use of<br />
poorly designed primer pairs, or heating/cooling steps that are too<br />
lengthy—nonspecific annealing <strong>and</strong> subsequent amplification may<br />
be observed. Figure 2B demonstrates this nonspecific annealing<br />
<strong>and</strong> amplification effect.<br />
A B<br />
‡ Fig. 2: Polyacrylamide gel analysis<br />
A highly specific amplified <strong>PCR</strong> product (A) is compared to (B)—<br />
a similar gel image showing more than one specific b<strong>and</strong>, which<br />
indicates nonspecific products.
Product highlights—Mastercycler® ep realplex<br />
Impulse <strong>PCR</strong>: a novel, accelerated <strong>and</strong> improved q<strong>PCR</strong> Hot Start method, continued.<br />
In real-<strong>time</strong> <strong>PCR</strong> assays for which SYBR ® Green I is the chosen<br />
fluorescent reporter molecule, nonspecific amplification may be<br />
indicated—not by additional b<strong>and</strong>s, but by a higher-than-accurate<br />
level of total fluorescence in the sample. And higher fluorescence<br />
resulting from the presence of nonspecific product means that<br />
accurate quantification is no longer possible. A view of the melt-<br />
ing curve plot of SYBR Green real-<strong>time</strong> <strong>PCR</strong> assays confirms the<br />
presence of nonspecific product through the appearance of more<br />
than one peak (Fig. 3), comparable to the single vs. multiple b<strong>and</strong>s<br />
shown in Fig. 2 on the previous page.<br />
‡ Fig. 3: Melting curve analysis profile of the final melting<br />
curve step across a number of samples of a real-<strong>time</strong> <strong>PCR</strong><br />
assay, each with SYBR Green I as the reporter dye<br />
Double-str<strong>and</strong>ed DNA-binding dyes such as SYBR Green I bind<br />
to any double-str<strong>and</strong>ed DNA molecule; therefore, it is important<br />
to design a highly specific assay that will eliminate the presence/<br />
amplification of nonspecific product.<br />
Blue = Impulse <strong>PCR</strong> Red = St<strong>and</strong>ard <strong>PCR</strong><br />
100<br />
90<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
Time<br />
realplex highlights<br />
The Impulse <strong>PCR</strong> option of realplex S silver block models is<br />
an <strong>Eppendorf</strong> invention. Impulse <strong>PCR</strong> acts as a “device-driven<br />
Hot Start” to greatly increase the specificity of real-<strong>time</strong> <strong>PCR</strong><br />
reactions <strong>and</strong>, thus, increase the dependability of SYBR Green<br />
dye experiments.<br />
The Impulse function targets the first initial ramping step of<br />
the real-<strong>time</strong> <strong>PCR</strong> program—identified earlier as a point of high<br />
risk for nonspecific amplification—by jump-starting the reaction<br />
with additional speed (up to 8 °C/s), thereby reaching the initial<br />
denaturation step much faster. The <strong>time</strong> for, <strong>and</strong> the possibility of,<br />
nonspecific annealing is minimized because samples spend very<br />
little <strong>time</strong> below their ideal programmed annealing temperature.<br />
‡ Fig. 4: Temperature ramping profiles of two experiments<br />
One uses Impulse <strong>PCR</strong> (in blue) <strong>and</strong> the other (in red) does not.<br />
The plot demonstrates <strong>time</strong> (x axis) versus temperature (y axis).<br />
This example shows that by selecting the Impulse <strong>PCR</strong> function,<br />
the sample reaches the initial denaturation temperature faster,<br />
which leads to a decrease in total run <strong>time</strong> <strong>and</strong> increased<br />
specificity—a device-driven Hot Start.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
37<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> | Instruments
Instruments | <strong>Real</strong>-<strong>time</strong> <strong>PCR</strong><br />
38<br />
realplex highlights<br />
Product highlights—Mastercycler® ep realplex<br />
High-speed, real-<strong>time</strong> <strong>PCR</strong> assay design for realplex silver block models<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> is an ideal application for the design <strong>and</strong> operation<br />
of high-speed <strong>PCR</strong> programs—providing results in minutes, rather<br />
than hours. Furthermore, in comparison to conventional <strong>PCR</strong>,<br />
complex post-<strong>PCR</strong> h<strong>and</strong>ling is no longer required, which reduces<br />
analysis <strong>time</strong> <strong>and</strong> further reduces overall <strong>time</strong> spent on each<br />
experiment. With Mastercycler ep realplex silver block models<br />
realplex 2 S <strong>and</strong> realplex 4 S, the already fast ramp rates of the<br />
aluminum block models increase from 4 ºC/3 ºC to 6 ºC/4.5 ºC<br />
(heating/cooling). Table 1 below lists a comparison of heating ramp<br />
rates among Mastercycler ep realplex <strong>and</strong> other real-<strong>time</strong> systems<br />
currently available.<br />
The key benefits of realplex’s silver block for ultra-high-speed<br />
real-<strong>time</strong> <strong>PCR</strong> are:<br />
‡ Visualize results in real <strong>time</strong> during a <strong>PCR</strong> run: at every cycle, the<br />
fluorescence of each sample chronologically appears <strong>and</strong> can be<br />
observed <strong>and</strong> analyzed—no more waiting for > 30 cycles, followed<br />
by post-<strong>PCR</strong> analysis!<br />
‡ Shorten total run <strong>time</strong>s: because longer amplicons require<br />
longer extension/elongation dwell <strong>time</strong>s, the total run <strong>time</strong> of<br />
<strong>PCR</strong> programs with shorter amplicons reap the greatest benefit<br />
from our ultra-high-speed ramping; <strong>and</strong> since amplicons under<br />
100 bp are optimal for real-<strong>time</strong> <strong>PCR</strong> applications, realplex S<br />
models, therefore, have a great impact on the reduction of total<br />
run <strong>time</strong>.<br />
Instrument Ramp rate—heating<br />
Mastercycler ep realplex S 6 °C/sec<br />
Mastercycler ep realplex 4 °C/sec<br />
Instrument M 3 °C/sec<br />
Instrument S 2.5 °C/sec<br />
Instrument A 1.6 °C/sec<br />
‡ Table 1: Ramp rates of several real-<strong>time</strong> <strong>PCR</strong> systems<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
‡ Fast optical detection also reduces total run <strong>time</strong>: realplex optical<br />
detection requires only 8 seconds for 1 to 2 channels or up to 16<br />
seconds for 4 channels—across an entire plate in a single cycle.<br />
‡ Optimized plate design: to supplement realplex’s very fast<br />
ramping, <strong>Eppendorf</strong> ® twin.tec <strong>PCR</strong> plates facilitate high-speed<br />
temperature change by providing optimal heat transfer through<br />
their thin well walls.<br />
The amplification plots in Fig. 1 on the next page show a<br />
dramatic reduction of total run <strong>time</strong> <strong>and</strong> total reaction volume on<br />
the Mastercycler ep realplex real-<strong>time</strong> <strong>PCR</strong> systems, while offering<br />
outst<strong>and</strong>ing reproducibility <strong>and</strong> peak performance.<br />
Would you base your life’s work on anything else?<br />
Keep in mind, the right chemistry also makes a difference:<br />
Hot Start polymerases that do not require lengthy activation<br />
steps of 10 minutes or more will not require any more <strong>time</strong> for<br />
initial denaturation than is needed by the template. Furthermore,<br />
the enzyme you choose must perform efficiently during<br />
short protocols.
Product highlights—Mastercycler® ep realplex<br />
High-speed, real-<strong>time</strong> <strong>PCR</strong> assay design for realplex silver block models, continued.<br />
A B<br />
Fig. 1<br />
Av.Ct 22.62<br />
St.dev. 0.05<br />
1 h 12 min<br />
C D<br />
Av.Ct 22.45<br />
St.dev. 0.10<br />
35 min 37 sec<br />
‡ Fig. 1: High-speed, real-<strong>time</strong> <strong>PCR</strong> using Mastercycler ep realplex 4 S<br />
Initial denaturation/<br />
activation<br />
40 cycles<br />
Denaturation Annealing/extension<br />
95 °C 95 °C 60 °C<br />
realplex highlights<br />
High reproducibility is demonstrated with a minimum of 15 replicates of a SRY TaqMan ® assay across a variety of <strong>PCR</strong> protocols <strong>and</strong> a variety<br />
of total reaction volumes (as low as 10 µl) with low st<strong>and</strong>ard deviation. (A) through (D) show the effect of a stepwise <strong>time</strong> optimization (see<br />
Table 2 below) of <strong>PCR</strong> profiles <strong>and</strong> concomitant reduction of reaction volumes on total <strong>PCR</strong> run <strong>time</strong>s <strong>and</strong> st<strong>and</strong>ard deviation—the reaction<br />
was reduced from 72 min down to 23 min 34 s.<br />
Total run <strong>time</strong> on a<br />
Mastercycler ep S realplex system<br />
A 2 min 15 s 60 s 72 min (50 µl reaction vol., tube control)<br />
B 2 min 10 s 20 s 42 min 02 s (20 µl reaction vol., tube control)<br />
C 20 s 3 s 20 s 35 min 37 s (20 µl reaction vol., tube control)<br />
D 20 s 3 s 17 s 23 min 34 s (10 µl reaction vol., block control)<br />
‡ Table 2: Optimization of the <strong>PCR</strong> profiles shown in Fig. 1<br />
Av.Ct 22.82<br />
St.dev. 0.08<br />
42 min<br />
Av.Ct 23.545<br />
St.dev. 0.08<br />
23 min 34 sec<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
39<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> | Instruments
Instruments | <strong>Real</strong>-<strong>time</strong> <strong>PCR</strong><br />
40<br />
realplex highlights<br />
Product highlights—Mastercycler® ep realplex<br />
High-speed, real-<strong>time</strong> <strong>PCR</strong> assay design for realplex silver block models, continued.<br />
General recommendations for the optimization of high-speed,<br />
real-<strong>time</strong> <strong>PCR</strong> protocols<br />
Since the “early days” of real-<strong>time</strong> <strong>PCR</strong>, 90 minutes to 2 hours<br />
has generally been the st<strong>and</strong>ard total run <strong>time</strong> required for a single<br />
st<strong>and</strong>ard assay. Lately there has been a real push to optimize<br />
reactions towards high-speed, real-<strong>time</strong> <strong>PCR</strong> protocols for<br />
increased sample throughput—providing the opportunity for more<br />
researchers to perform many more types <strong>and</strong> quantities of assays<br />
on a single real-<strong>time</strong> <strong>PCR</strong> device.<br />
Until now, total reaction <strong>time</strong> for high-speed q<strong>PCR</strong> has typically<br />
been 40 minutes—a substantial <strong>time</strong>-savings when compared<br />
to st<strong>and</strong>ard assays. With the introduction of Mastercycler ep<br />
realplex S systems, we have further pushed the limits of speed<br />
<strong>and</strong> shortened real-<strong>time</strong> <strong>PCR</strong> reactions to just over 20 minutes!<br />
With real-<strong>time</strong> <strong>PCR</strong> this fast, many more labs will want to take<br />
high-speed assay optimization into serious consideration.<br />
Some general recommendations for the stepwise optimization<br />
of high-speed, real-<strong>time</strong> <strong>PCR</strong> are explained here <strong>and</strong> on the next<br />
page. By following <strong>Eppendorf</strong>’s general optimization tips <strong>and</strong><br />
adopting the reliability <strong>and</strong> speed of Mastercycler ep realplex 2 S<br />
or realplex 4 S, you can routinely approach total run <strong>time</strong>s of 24<br />
minutes or less.<br />
Forward/reverse<br />
primer concentration<br />
50 nM<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
100 nM<br />
300 nM<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Step 1: Examine your target length<br />
Consistent with the design of most TaqMan ® assays, an amplifica-<br />
tion target should not exceed a length of 100 bp by much—due to<br />
the fact that a <strong>PCR</strong> program’s extension <strong>time</strong> can be significantly<br />
reduced for <strong>PCR</strong> targets of such short lengths. The target length of<br />
the <strong>PCR</strong> reaction from the amplification plots shown in Fig. 1 (page<br />
39) was 80 bp.<br />
Step 2: Optimize primer design<br />
Good primer design is an important variable for highly efficient<br />
<strong>and</strong> robust <strong>PCR</strong>.<br />
(a) The presence of self-complementary structures within the<br />
primer (<strong>and</strong> probe) flanking regions should be prevented to avoid<br />
nonspecific <strong>PCR</strong> products, resulting in loss of <strong>PCR</strong> efficiency <strong>and</strong><br />
possible false-positive results (SYBR ® Green I).<br />
(b) It is helpful to design primers with a melting temperature (T m)<br />
of 60 °C or higher so that two-step <strong>PCR</strong> programs may be used<br />
(see step 5).<br />
Note: A useful <strong>and</strong> widely available tool for primer design that<br />
considers the above-mentioned general guidelines is Primer3-<br />
Software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi)<br />
[Steve Rozen <strong>and</strong> Helen J. Skaletsky (2000) Primer3 on the WWW<br />
for general users <strong>and</strong> for biologist programers. Krawetz S, Misener<br />
S (eds) Bioinformatics Methods <strong>and</strong> Protocols: Methods in<br />
Molecular Biology. Humana Press, Totowa, NJ, pp 365-386].<br />
600 nM<br />
900 nM<br />
50 nM 50/50 100/50 300/50 600/50 900/50<br />
100 nM 50/100 100/100 300/100 600/100 900/100<br />
300 nM 50/300 100/300 300/300 600/300 900/300<br />
600 nM 50/600 100/600 300/600 600/600 900/600<br />
900 nM 50/900 100/900 300/900 600/900 900/900<br />
‡ Table 3: A common primer-matrix for suggested optimization of forward <strong>and</strong> reverse primer combinations
Product highlights—Mastercycler® ep realplex<br />
High-speed, real-<strong>time</strong> <strong>PCR</strong> assay design for realplex silver block models, continued.<br />
Step 3: Gradient real-<strong>time</strong> <strong>PCR</strong> optimization<br />
A gradient <strong>PCR</strong> should be performed to find the optimal<br />
annealing/extension temperature for the primers. This step is easy,<br />
yet critically important for achieving the best primer performance in<br />
any <strong>PCR</strong> reaction.<br />
Note: Detailed instruction on the gradient function of realplex <strong>and</strong><br />
its importance for real-<strong>time</strong> <strong>PCR</strong> optimization—including two-step<br />
protocols—appears on page 34.<br />
Step 4: Primer matrix optimization<br />
A primer-matrix that spans a range of common concentrations<br />
of the forward <strong>and</strong> reverse primer should be performed to find<br />
the optimal combination of concentrations for both primers.<br />
A commonly used primer-matrix is featured in Table 3 on the<br />
previous page.<br />
Note: To achieve the most reliable results, each combination should<br />
be performed with 3 replicates.<br />
Step 5: Design a two-step program for added speed<br />
A two-step <strong>PCR</strong> program with a combined annealing/extension<br />
step of 60 °C or higher can be easily optimized by activating<br />
realplex’s gradient option in the second cycle step. A two-step<br />
program enables you to achieve the fastest run <strong>time</strong>s because<br />
it features fewer ramping steps than a conventional three-step<br />
protocol.<br />
(a) To begin optimization of a two-step protocol, it is good to start<br />
with a default program such as that listed in Fig. 1 <strong>and</strong> Table 2 on<br />
page 39.<br />
realplex highlights<br />
(b) Change the annealing/extension temperature to the optimal<br />
temperature as explained in Step 3.<br />
(c) Based on the length of the target amplicon for your assay (see<br />
Step 1 on the previous page), shorten the annealing/extension dwell<br />
<strong>time</strong> <strong>and</strong> test to confirm high performance.<br />
(d) Next, consider decreasing the cycle denaturation dwell <strong>time</strong>.<br />
As a general rule, if your template’s GC-content is < 50%, shorter<br />
<strong>time</strong>s for denaturation should be sufficient.<br />
(e) Consider the gradient option to test a range of denaturation<br />
temperatures in the event that templates are GC-rich—because<br />
GC-rich templates may not amplify sufficiently at shorter<br />
denaturation dwell <strong>time</strong>s. Again, choose your denaturation dwell<br />
<strong>time</strong> <strong>and</strong> perform an assay test to confirm good performance.<br />
(f) Decrease your total reaction volume. Lower volumes require less<br />
<strong>time</strong> to heat <strong>and</strong>, therefore, less overall <strong>time</strong> to amplify. Perform a<br />
melting curve analysis to confirm high specificity.<br />
Note: If working with volumes of 10 µl or higher, “Simulate tube”<br />
mode in your program Header may be required. Block mode is<br />
faster <strong>and</strong> works well with low-volume samples (10 µl).<br />
(g) Shorten your initial denaturation. If GC-content is < 50% <strong>and</strong><br />
your mastermix polymerase enables short activation <strong>time</strong>s, follow<br />
this guide’s recommendations for a short initial denaturation.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
41<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> | Instruments
Instruments | <strong>Real</strong>-<strong>time</strong> <strong>PCR</strong><br />
42<br />
realplex highlights<br />
Product highlights—Mastercycler® ep realplex<br />
Benefits of realplex’s homogeneity <strong>and</strong> accuracy on reproducibility in real-<strong>time</strong> q<strong>PCR</strong><br />
A key contributor to reproducible real-<strong>time</strong> <strong>PCR</strong> is the accuracy<br />
<strong>and</strong> uniformity of temperatures across a range of samples. Ideally,<br />
thermal block-based q<strong>PCR</strong> systems must demonstrate an even<br />
temperature profile across the entire block, with identical heating<br />
<strong>and</strong> cooling speeds <strong>and</strong> dwell temperatures in every position.<br />
Without this high degree of temperature control, edge effects<br />
may occur, reflecting a loss of temperature to the environment<br />
at the outer edges of the block.<br />
Addressing this challenge—<strong>and</strong> increasing the reproducibility of<br />
real-<strong>time</strong> <strong>PCR</strong> assays across a 96-well plate—is <strong>Eppendorf</strong>’s Triple<br />
Circuit Technology. This unique arrangement of multiple Peltier<br />
elements into three defined temperature control regions provides<br />
the following key benefits: (a) more linear gradient testing of optimal<br />
annealing temperature or two-step <strong>PCR</strong> optimization, translating to<br />
more distinct temperatures tested in a single experiment, <strong>and</strong> (b) a<br />
higher degree of temperature control around the outer regions of<br />
the block, thus decreasing edge effects <strong>and</strong> allowing the effective<br />
use of all wells in a 96-well plate with high reproducibility.<br />
Mastercycler Triple Circuit Technology benefits<br />
‡ Ensures precise control of<br />
the temperature gradient<br />
‡ Enhances linearity of<br />
the gradient<br />
‡ The thermal block is controlled by three separate<br />
temperature control circuits<br />
Order direct from <strong>Eppendorf</strong> North America.<br />
‡ Reduces temperature<br />
loss in the peripheral areas<br />
during uniform (nongradient)<br />
temperature mode<br />
Critical impact<br />
realplex’s combined optical <strong>and</strong> temperature control features<br />
provide uniform temperature <strong>and</strong> light signals for all positions in a<br />
plate-based q<strong>PCR</strong> assay. The following amplification plot (Fig. 1)<br />
shows convincing results: 96 samples with perfect replicate results,<br />
an excellent foundation for the success of any real-<strong>time</strong> <strong>PCR</strong><br />
application or assay.<br />
9000<br />
8000<br />
7000<br />
6000<br />
5000<br />
4000<br />
3000<br />
Fluorescence (norm) 10000<br />
2000<br />
1000<br />
0<br />
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30<br />
Cycle<br />
Threshold: 186 (Noiseb<strong>and</strong>)<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
‡ Fig. 1: Mastercycler ep realplex’s block <strong>and</strong> optical<br />
Baseline settings: automatic, Drift correction OFF<br />
homogeneity provide high reproducibility across 96 replicates
Product highlights—Mastercycler® ep realplex<br />
Optical concept highlight – 96 well excitation<br />
Mastercycler ep realplex light path<br />
� Light emission from 96 LEDs (470 nm)<br />
�a/b/c Focusing with 96-lens-arrays<br />
� Semipermeable beam splitter<br />
� Light emission from excited fluores-<br />
Optical design<br />
An additional requisite of reproducible real-<strong>time</strong> <strong>PCR</strong> is the design<br />
of a uniform light source for even well-to-well excitation—in other<br />
words, the excitation source should show the same intensity in<br />
every position. To this end, realplex features a 96-LED array for<br />
excitation, <strong>and</strong> the intensity of each LED is normalized to a mean<br />
value. Thus, each sample receives the same light intensity, <strong>and</strong><br />
every well has a direct light source—with stray light minimized.<br />
This is in stark contrast to halogen-based lamps that decrease in<br />
intensity over their lifespan <strong>and</strong>, thus, may negatively impact results<br />
over <strong>time</strong>.<br />
cent dyes in the reaction mixture<br />
(see representation in the graphic)<br />
� Merging of the rays through a<br />
“96-in-1” optical fiber<br />
� Focusing; passage through additional<br />
beam splitters<br />
� Filter wheel with interference filters<br />
� Advancing the light beam to the<br />
photomultiplier<br />
‡ Inside view of the<br />
realplex’s heated lid <strong>and</strong><br />
96-LED array<br />
realplex highlights<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
43<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> | Instruments
Consumables | <strong>PCR</strong><br />
44<br />
<strong>PCR</strong> Consumables<br />
twin.tec <strong>PCR</strong> plates<br />
Product features<br />
‡ One-piece design<br />
combines polycarbonate<br />
<strong>and</strong> polypropylene for<br />
optimum performance<br />
‡ Increased plane parallelism<br />
‡ Extremely thin-walled<br />
for optimum heat transfer<br />
to the sample<br />
‡ Low-profile design enhances<br />
efficiency of <strong>PCR</strong> <strong>and</strong> enables<br />
the highest efficiency for small<br />
sample volumes<br />
‡ Raised well rims for<br />
effective sealing, also reduces<br />
risk of cross-contamination<br />
‡ Improved well-to-well<br />
tolerance<br />
Ordering information<br />
‡ Autoclavable<br />
(121 °C, 20 min)<br />
‡ Certified to be free of<br />
any detectable human DNA,<br />
DNase, RNase <strong>and</strong><br />
<strong>PCR</strong> inhibitors*<br />
‡ Meets SBS footprint<br />
recommendations<br />
‡ Max. well volumes:<br />
– 45 µl, twin.tec 384<br />
– 150 µl, twin.tec 96 skirted—<br />
when used with cap strips<br />
– 250 µl, twin.tec 96 semi-<br />
skirted—ideal for q<strong>PCR</strong> on<br />
a variety of systems<br />
* Certificate, test procedures <strong>and</strong> detailed<br />
information available upon request.<br />
Description Catalog No.<br />
twin.tec <strong>PCR</strong> Plate 96, skirted (clear wells), 25 pcs.<br />
Clear 951020401<br />
Yellow 951020427<br />
Green 951020443<br />
Blue 951020460<br />
Red 951020486<br />
twin.tec <strong>PCR</strong> Plate 96, skirted (black wells), 25 pcs.<br />
Yellow (not shown) 951020508<br />
twin.tec <strong>PCR</strong> Plate 96, semiskirted (frosted wells), 25 pcs.<br />
Clear 951020303<br />
Yellow 951020320<br />
Green 951020346<br />
Blue 951020362<br />
Red 951020389<br />
twin.tec <strong>PCR</strong> Plate 384, skirted (colorless wells), 25 pcs.<br />
Clear 951020702<br />
Yellow 951020711<br />
Green 951020729<br />
Blue 951020737<br />
Red 951020745<br />
Order from your laboratory supply dealer or <strong>Eppendorf</strong> North America.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
twin.tec plates are made of the best quality materials for use<br />
manually AND with automated systems: virgin polypropylene<br />
ensures minimal binding of DNA, RNA <strong>and</strong> enzymes—with<br />
maximum recovery; polycarbonate, which provides sturdiness<br />
<strong>and</strong> high mechanical stability, makes up the plate surface <strong>and</strong><br />
frame. The well walls are 20% thinner than conventional thin-<br />
walled tubes, providing optimal heat transfer between the block<br />
<strong>and</strong> your samples. Choose from 3 styles (unskirted, skirted <strong>and</strong><br />
semiskirted) <strong>and</strong> 5 colors to suit your experimental needs <strong>and</strong><br />
ease identification/h<strong>and</strong>ling.
twin.tec real-<strong>time</strong> <strong>PCR</strong> plates*<br />
<strong>Eppendorf</strong> twin.tec real-<strong>time</strong> <strong>PCR</strong> plates* offer all the advantages of<br />
regular twin.tec plates <strong>and</strong> give you the advantage of white wells for<br />
your real-<strong>time</strong> <strong>PCR</strong>.<br />
The limiting factor in low volume real-<strong>time</strong> <strong>PCR</strong> is often the<br />
remaining intensity of fluorescence. The white wells of the<br />
<strong>Eppendorf</strong> twin.tec real-<strong>time</strong> <strong>PCR</strong> plates* reflect fluorescence<br />
much better than clear or frosted wells. Thus, lower levels of<br />
fluorescence are still detectable with the same instrument –<br />
just by using the right consumables.<br />
Product features<br />
‡ White wells for better reflection<br />
‡ High mechanical stability<br />
‡ Raised rims for effective sealing<br />
‡ “Skirted” (stackable) <strong>and</strong> “semi-skirted” plates<br />
‡ Optimal heat transfer due to reduced wall thickness<br />
‡ Autoclavable (121 °C, 20 min.)<br />
Ordering information<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> Consumables<br />
Additionally, white wells significantly reduce interfering background<br />
fluorescence <strong>and</strong> lead to increased homogeneity of replicates <strong>and</strong><br />
reproducible results.<br />
The rigidity of the polycarbonate frame ensure easy <strong>and</strong> reliable<br />
h<strong>and</strong>ling – manually or automated. The polypropylene wells<br />
guarantee excellent <strong>and</strong> fast temperature transfer to the sample.<br />
‡ The intensity of fluorescence measured by the instrument<br />
is up to 10-fold higher than with frosted wells.<br />
Description Catalog No.<br />
twin.tec 96 real-<strong>time</strong> <strong>PCR</strong> plates*, skirted white, set of 25 pcs. 951022015<br />
twin.tec 96 real-<strong>time</strong> <strong>PCR</strong> plates*, skirted blue, set of 25 pcs. 951022003<br />
twin.tec 96 real-<strong>time</strong> <strong>PCR</strong> plates*, skirted black, set of 25 pcs. 951022027<br />
twin.tec 96 real-<strong>time</strong> <strong>PCR</strong> plates*, semi-skirted white, set of 25 pcs. 951022055<br />
twin.tec 96 real-<strong>time</strong> <strong>PCR</strong> plates*, semi-skirted blue, set of 25 pcs. 951022043<br />
twin.tec 96 real-<strong>time</strong> <strong>PCR</strong> plates*, semi-skirted black, set of 25 pcs. 951022067<br />
*<strong>Eppendorf</strong> owns protective rights under European Patent EP 1 161 994, US patents pending.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
45<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> | Consumables
Consumables | Instruments | <strong>PCR</strong><br />
46<br />
<strong>PCR</strong> Accessories <strong>and</strong> Consumables<br />
Heat sealing materials<br />
Heat Sealer product features<br />
‡ Safe <strong>and</strong> easy hermetic heat<br />
sealing of 96- <strong>and</strong> 384-well<br />
plates (384-well base available<br />
separately)<br />
‡ Eliminates evaporation<br />
in <strong>PCR</strong>, reducing cross-<br />
contamination<br />
‡ Suitable for plates of<br />
different heights<br />
‡ Optimum sealing with<br />
<strong>Eppendorf</strong> ® Heat Sealing<br />
Foils <strong>and</strong> Films at a preset<br />
temperature<br />
Film/Foil product features<br />
‡ Hermetic sealing of<br />
multiwell plates, especially<br />
recommended for low<br />
reaction volumes<br />
‡ Best protection against<br />
evaporation during <strong>PCR</strong><br />
‡ Certified free of human<br />
DNA, DNase, RNase <strong>and</strong><br />
<strong>PCR</strong> Inhibitors*<br />
Technical specifications<br />
‡ Compact <strong>and</strong> portable, ideal<br />
for transporting <strong>and</strong> storing<br />
samples<br />
‡ Integrated thermostat<br />
prevents overheating<br />
‡ Heating plate recessed<br />
for safety<br />
‡ Use with optically clear<br />
heat-sealing film for real-<strong>time</strong><br />
q<strong>PCR</strong> applications<br />
‡ Heat sealing film is optically<br />
clear for real-<strong>time</strong> <strong>PCR</strong><br />
applications.<br />
* Certificate, test procedures <strong>and</strong> detailed<br />
information available upon request.<br />
Order from your laboratory supply dealer or <strong>Eppendorf</strong> North America.<br />
Ordering information<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Description Catalog No.<br />
Heat Sealer, 115 V/50 Hz 951023078<br />
Base plate, for 384-well plate 951023086<br />
Heat Sealing Film, 10 x 10 pcs. 951023060<br />
Peel-it-lite Foil, 100 pcs. 951023205<br />
Pierce-it-lite Foil, 100 pcs. 951023213<br />
Foil Stripper 951023043<br />
Heat Sealing Film Peel-it-lite Foil Pierce-it-lite Foil<br />
Packaging: 10 x 10 pcs. 1 x 100 pcs. 1 x 100 pcs.<br />
Features: Optically clear polyester/<br />
polypropylene laminate<br />
Extremely stable sealing option<br />
—cannot be removed or pierced<br />
Laminated aluminum foil<br />
Easily removable<br />
Laminated aluminum foil<br />
Easily pierced—even with<br />
multichannel pipettes<br />
No glue residue on the<br />
pipette tips<br />
Seal integrity: –80 °C to 140 °C –200 °C to 120 °C –80 °C to 120 °C<br />
Sealing <strong>time</strong> with<br />
<strong>Eppendorf</strong> Heat Sealer:<br />
1–2 s 2–4 s 2–3 s<br />
Weldable materials: Polypropylene Polypropylene<br />
Polyethylene<br />
Special applications: Colorimetric applications<br />
Fluorescence applications,<br />
including real-<strong>time</strong> <strong>PCR</strong><br />
Storage of hazardous samples<br />
Storage at extremely low<br />
temperatures (–200 °C)<br />
Can even be removed at –80 °C<br />
Plate can be resealed<br />
(after removal of old foil)<br />
Polypropylene<br />
<strong>PCR</strong> with water bath cyclers<br />
Storage <strong>and</strong> transport<br />
of samples
Applications<br />
ARTS<br />
47
Applications | ARTS<br />
48<br />
Application notes<br />
<strong>Eppendorf</strong> offers more: application support<br />
Both automation <strong>and</strong> real-<strong>time</strong> <strong>PCR</strong> applications<br />
vary in their scope <strong>and</strong> complexity. This section<br />
includes application notes related to <strong>PCR</strong>/q<strong>PCR</strong><br />
<strong>and</strong> nucleic acid purification that highlight the<br />
Mastercycler ® ep realplex <strong>and</strong> epMotion ® systems—<br />
demonstrating their superior performance as st<strong>and</strong>alone<br />
devices as well as the great benefits of using<br />
them together. More application notes are available<br />
to view <strong>and</strong> download at www.eppendorfna.com/arts.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.
Automated genomic DNA purification<br />
Application notes<br />
Automated genomic DNA purification from tissue culture cells using the Promega<br />
Wizard ® SV 96 Genomic DNA Purification System <strong>and</strong> the <strong>Eppendorf</strong> epMotion ® 5075 VAC<br />
Sarah Shultz, Promega Corporation, Madison, WI<br />
Abstract<br />
Purification of genomic DNA from tissue culture cells in a high-<br />
throughput method is essential for many downstream applications<br />
in molecular biology. In this report, we have developed an auto-<br />
mated method to meet the need for high-throughput genomic DNA<br />
purification on the <strong>Eppendorf</strong> ® epMotion ® 5075 VAC. Genomic<br />
DNA is purified from tissue culture cells with no detectable<br />
cross-contamination during the automated isolation procedure.<br />
Genomic DNA is then analyzed for yield <strong>and</strong> purity by measuring<br />
sample absorbance at 260 nm <strong>and</strong> 280 nm, amplified to confirm<br />
compatibility for downstream <strong>PCR</strong> amplification (1.2 kb IL-1ß), <strong>and</strong><br />
compared to genomic DNA extracted from the same tissue culture<br />
cells using a manual method.<br />
Introduction<br />
High-throughput isolation <strong>and</strong> purification of genomic DNA is<br />
an essential step for many researchers in molecular biology.<br />
Genomic DNA is traditionally purified from cultured cell samples<br />
by mechanically disrupting the cells by proteolysis, followed by<br />
phenol extraction. This process can be tedious, labor-intensive <strong>and</strong><br />
hazardous because of the use of toxic organic compounds. Thus,<br />
the development of a simple, h<strong>and</strong>s-free, high-throughput genomic<br />
DNA purification procedure capable of isolating high-quality DNA<br />
in a relatively short amount of <strong>time</strong> without contamination between<br />
samples is desirable.<br />
The Promega Wizard SV 96 Genomic DNA Purification System<br />
provides a high-throughput, membrane-based technique for<br />
consistent preparation of genomic DNA from tissue culture cells.<br />
Amplifiable genomic DNA can be isolated from up to 5×10 6 cells,<br />
without a centrifugation clearing step. The SV 96 genomic DNA<br />
isolation procedure involves sample lysate preparation, capture<br />
of genomic DNA, washing to remove impurities, <strong>and</strong> eluting the<br />
purified DNA.<br />
The <strong>Eppendorf</strong> epMotion 5075 VAC is a flexible automated<br />
pipetting system, that can be adapted to various liquid h<strong>and</strong>ling<br />
tasks. The concept of exchangeable tips <strong>and</strong> the “Free Jet<br />
Dispensing” technology enables this workstation to dispense<br />
liquids contact-free <strong>and</strong> makes it ideal for implementing complex<br />
work steps where precision <strong>and</strong> reliability are required.<br />
Materials <strong>and</strong> methods<br />
‡ <strong>Eppendorf</strong> epMotion 5075 VAC equipped as follows:<br />
‡ Gripper<br />
‡ Dispensing tools TM1000-8 <strong>and</strong> TM300-8<br />
‡ Vacuum with manifold<br />
‡ Reservoir Rack<br />
‡ Height spacers 85 mm <strong>and</strong> 35 mm<br />
‡ Vac Frame 2<br />
‡ Waste Tub<br />
‡ <strong>Eppendorf</strong> consumables<br />
‡ 5 x 100 ml Reagent Reservoirs<br />
‡ 1 x 1000 μl epT.I.P.S. Motion Filtered Tips<br />
‡ 1 x 300 μl epT.I.P.S. Motion Filtered Tips<br />
‡ Promega Wizard SV 96 Genomic DNA Purification System<br />
‡ Promega 1.2 ml 96-Well Round-Bottom Deep Well Plate<br />
(Elution Plate)<br />
‡ 96-Well Tissue Culture Plate (Sample Plate)<br />
‡ Fresh tissue culture cells<br />
‡ Sterile 1x Phosphate Buffered Saline (PBS)<br />
Cell <strong>and</strong> reagent preparation<br />
Two common tissue culture cell lines, HEK-293 <strong>and</strong> NIH-3T3,<br />
were prepared at 1x10 5 cells per well <strong>and</strong> placed in 96-Well Tissue<br />
Culture Plates (Corning, USA) in a checkerboard pattern, as shown<br />
in Figure 1.<br />
‡ Figure 1: Sample Plate checkerboard layout.<br />
Every other well was left empty to serve as a control for method<br />
cross-contamination. Reagents were prepared <strong>and</strong> dispensed into<br />
five 100 ml Reagent Reservoirs, as described in Table 1.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
49<br />
ARTS | Applications
Applications | ARTS<br />
50<br />
Application notes<br />
Automated genomic DNA purification<br />
Automated genomic DNA purification, continued.<br />
Reservoir Contents Comments<br />
20ml Wizard ® SV Lysis Buffer<br />
90ml Wizard ® SV Wash Solution with EtOH Add 95% EtOH<br />
to the Wizard ® SV<br />
90ml Wizard<br />
96 Wash Solution<br />
bottle as directed<br />
on the bottle label<br />
before dispensing.<br />
® SV Wash Solution with EtOH<br />
90ml Wizard ® SV Wash Solution with EtOH<br />
30ml Nuclease-Free Water<br />
‡ Table 1: Contents of the Reagent Reservoirs in the<br />
Reservoir Rack.<br />
Labware was placed onto the epMotion 5075 VAC worktable, as<br />
shown in Figure 2 <strong>and</strong> Table 2. The automated method was then<br />
started.<br />
‡ Figure 2: Screenshot from the epMotion Editor showing the<br />
epMotion 5075 VAC setup for the Automated Wizard SV 96<br />
Genomic DNA Tissue Culture Method.<br />
Position Labware<br />
A2 1000μl epTIPS Motion Filtered Tips<br />
A3 Reservoir rack with 5 Reagent Reservoirs<br />
A4 Empty<br />
B0 Empty<br />
B1 Empty<br />
B2 300μl epTIPS Motion Filtered Tips<br />
B3 Filter Plate sitting atop 85mm Height Spacer<br />
Vacuum Empty<br />
C1 Waste Tub with quarter wall separators<br />
C2<br />
Elution Plate: 96-Well 2.2ml Square-Bottom Deep<br />
Well Plate<br />
C3 Sample Plate: 96-Well Tissue Culture Plate<br />
C4 Vac Frame 2 atop 35mm Height Spacer<br />
‡ Table 2: epMotion 5075 Worktable setup details by position.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Automated method overview<br />
The automated method begins by dispensing 150 μl Lysis Buffer<br />
to each well of the Sample Plate. The dispensing tool mixes <strong>and</strong><br />
transfers the cell lysates to a Filter Plate atop the vacuum manifold<br />
apparatus. A vacuum is then applied <strong>and</strong> lysates are pulled through<br />
the Filter Plate. During this step, genomic DNA binds to the<br />
filter plate.<br />
The dispensing tool next dispenses 810 μl Wash Solution to each<br />
well of the Filter Plate. A vacuum is applied <strong>and</strong> the Wash Solution<br />
is pulled through the Filter Plate. This step is repeated for a total<br />
volume of 2.4 ml of Wash Solution per well. After washing, the<br />
vacuum remains on for an additional 10 minutes to remove any<br />
residual Wash Solution from the Filter Plate. Once the Filter Plate<br />
is dry, the Gripper tool disassembles the vacuum manifold stack<br />
by moving the Vac Frame 2 <strong>and</strong> Filter Plate from the manifold to a<br />
holding position. The Gripper tool next moves the Elution Plate into<br />
the vacuum manifold, to replace the Waste Tub. Then, the Gripper<br />
tool reassembles the vacuum manifold stack by moving the Vac<br />
Frame 2 <strong>and</strong> Filter Plate back top the vacuum.<br />
After reassembly, the dispensing tool transfers 200 μl Elution<br />
Buffer to each well of the Filter Plate. A final vacuum is applied <strong>and</strong><br />
the Elution Buffer is pulled through the Filter Plate while eluting<br />
genomic DNA. At the end of the automated method, purified<br />
genomic DNA from the original tissue culture cells is eluted into the<br />
Elution Plate. The total processing <strong>time</strong> for 96 samples using the<br />
automated method is just under 1 hour.<br />
Analysis of purified genomic DNA<br />
Downstream analyses of the purified genomic DNA samples were<br />
performed. One microliter samples from the Elution Plate were<br />
placed directly onto a ND-1000 Spectrophotometer (Nanodrop,<br />
USA). Average yield (A260) <strong>and</strong> purity (A260/A280) measurements<br />
were taken. For eluates purified from NIH-3T3 cells, 1 μl purified<br />
genomic DNA samples were also <strong>PCR</strong> amplified using Promega’s<br />
<strong>PCR</strong> Master Mix, Cat.# M712B (Promega, USA) <strong>and</strong> IL-1ß primers<br />
(Integrated DNA Technologies, USA). A negative control reaction<br />
comprised of Elution Buffer only was included in the analysis.<br />
The <strong>PCR</strong> thermal cycling conditions were as follows: 2 minutes<br />
at 94 °C; followed by 40 cycles of 94 °C for 30 seconds, 58 °C for<br />
30 seconds, <strong>and</strong> 72 °C for one minute; final extension at 72 °C for<br />
5 minutes. After <strong>PCR</strong> amplification, 10 μl of each reaction product<br />
was separated on a 1.2% agarose gel <strong>and</strong> visualized by ethidium<br />
bromide staining.<br />
For additional comparative analysis, the Promega Wizard SV 96<br />
Genomic DNA Purification System was also performed manually<br />
using HEK-293 tissue culture cells at 1x10 5 cells per well. Cells<br />
were prepared in the same checkerboard pattern as described<br />
above. Refer to Promega TB#303 for details on the manual<br />
processing procedure.
Automated genomic DNA purification<br />
Automated genomic DNA purification, continued.<br />
Results <strong>and</strong> Discussion<br />
Use of the automated method described in this article incorporating<br />
the Promega Wizard SV 96 Genomic DNA Purification System to<br />
isolate genomic DNA from tissue culture cells using the epMotion<br />
5075 VAC results in high-quality genomic DNA compatible with<br />
downstream <strong>PCR</strong> amplification. Successful amplification of IL-1ß<br />
from 1 μl purified genomic DNA samples isolated from NIH-3T3<br />
tissue culture cells is shown in Figure 3, lanes D4, D6, D8, <strong>and</strong> D10.<br />
‡ Figure 3: Genomic DNA isolated from NIH-3T3 tissue culture<br />
cells using the Wizard SV 96 gDNA Tissue Culture Method on<br />
the epMotion 5075 VAC. <strong>PCR</strong> products were amplified from<br />
1 μl of purified sample from each well for IL-1ß. Ten microliters of<br />
<strong>PCR</strong> product was run on a 1.2% agarose gel <strong>and</strong> visualized by<br />
staining with ethidium bromide. Expected <strong>PCR</strong> product for IL-1ß<br />
is approximately 1.2 kb, as shown in lanes D4, D6, D8, <strong>and</strong> D10.<br />
No <strong>PCR</strong> product is expected from blank samples, as shown in<br />
lanes D5, D7, D9, <strong>and</strong> D11, as well as in the negative control lane<br />
–C which contained only Elution Buffer. A marker st<strong>and</strong>ard was<br />
included for size estimation, lane M (Promega BenchTop 1 kb<br />
DNA Ladder).<br />
Ten microliters of each <strong>PCR</strong> product was run on a 1.2% agarose<br />
gel <strong>and</strong> visualized by staining with ethidium bromide. No cross-<br />
contamination between wells of the same Elution Plate was<br />
observed, as shown on lanes D5, D7, D9, <strong>and</strong> D11. A negative<br />
control consisting of amplified Elution Buffer shows no amplified<br />
product in lane –C, as expected. A marker st<strong>and</strong>ard lane M,<br />
BenchTop 1 kb DNA Ladder (Promega, USA), was included for<br />
size estimation. Expected <strong>PCR</strong> products size for IL-1ß is<br />
approximately 1.2 kb.<br />
1x10 5 293 cells (n=12)<br />
Blank Wells (n=12)<br />
Application notes<br />
Genomic DNA purified using this automated method is also of<br />
quality yield <strong>and</strong> purity, as determined by spectrophotometer<br />
absorbance measurements at 260 nm <strong>and</strong> 280 nm. In addition,<br />
DNA yields are comparable to those prepared manually. This can<br />
be seen in the data presented in Table 3. Spectrophotometer<br />
measurements collected from 24 genomic DNA samples processed<br />
using the automated <strong>and</strong> manual methods isolated from HEK-<br />
293 tissue culture cells show comparatively equal yields between<br />
methods from wells containing 1x105 cells (1.0 ± 0.2 μg <strong>and</strong> 0.9 ±<br />
0.2 μg, respectively). Expected average DNA yield is approximately<br />
0.8 μg for the 293 cell line at this density. Spectrophotometer<br />
measurements also display excellent genomic DNA purity (A260/<br />
A280) for both methods (1.8 ± 0.1 <strong>and</strong> 1.9 ± 0.2, respectively).<br />
Expected average purity (A260/A280) is ≥ 1.7. Finally, further<br />
support that no cross-contamination exists between wells of the<br />
same Elution Plate was evidenced in Table 3, as the average DNA<br />
yield for both Blank wells groups analyzed were approximately zero<br />
(0.1 ± 0.1 μg <strong>and</strong> 0.0 ± 0.1 μg, respectively).<br />
This simple, high-throughput, automated genomic DNA purification<br />
procedure on the epMotion 5075 VAC successfully isolates high-<br />
quality DNA in less than one hour without contamination between<br />
samples. This method nicely fits the needs of researchers requiring<br />
considerable sample sets of genomic DNA for downstream<br />
applications in molecular biology.<br />
Automated Manual<br />
DNA (µg) 260/280 ng/µl DNA (µg) 260/280 ng/µl<br />
AVG 1.0 1.8 4.5 0.9 1.9 4.1<br />
STDEV 0.2 0.1 1.1 0.2 0.2 0.9<br />
AVG 0.1 1.0 0.3 0.0 0.1 -0.2<br />
STDEV 0.1 2.2 0.3 0.1 0.6 0.5<br />
‡ Table 3: Spectrophotometer measurements collected from 24 samples processed using the Automated Wizard SV 96 Genomic DNA<br />
Tissue Culture Method on the epMotion 5075 VAC <strong>and</strong> manual Wizard SV 96 Genomic DNA Purification System. Expected average DNA<br />
yield from wells containing 1x105 293 cells is approximately 0.8 μg. Expected average purity (A260/A280) is ≥ 1.7.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
51<br />
ARTS | Applications
Applications | ARTS<br />
52<br />
Application notes<br />
Isolation of high quality BAC DNA<br />
Isolation of high quality BAC DNA using the Perfectprep ® BAC 96 Kit from 5 PRIME on the<br />
epMotion ® 5075 VAC from <strong>Eppendorf</strong><br />
Sabine Mueller, Ph.D., 5 PRIMe GmbH, Renate Froendt, eppendorf <strong>AG</strong>, Hamburg, Germany<br />
Abstract<br />
<strong>Eppendorf</strong>’s continuing efforts to provide complete systems for<br />
biological research have created a system for the automated<br />
isolation of BAC DNA. This system uses the 5 PRIME Perfectprep<br />
BAC 96 Kit on the <strong>Eppendorf</strong> epMotion 5075 VAC allowing for the<br />
simultaneous, fully automated isolation of BAC DNA from 96 clones.<br />
The typical yield of a single BAC-clone from a 96-clone library plate<br />
was 0.5 – 1.0 µg, <strong>and</strong> the average Phred Q>20 score of one library<br />
plate was 676 bases with a passing rate of 100% (scores >100<br />
bases). Consistent results were obtained when isolating a single<br />
BAC clone from all 384 wells of four 96-well culture plates. The<br />
average Phred Q>20 score of 192 of these samples was 718 bases<br />
with a passing rate of 97.4%. The "walk away" protocol requires<br />
approximately 75 minutes <strong>and</strong> results in highly pure BAC DNA<br />
with sufficient yields for five downstream applications such as four<br />
sequencing reactions <strong>and</strong> one fingerprinting analysis.<br />
Introduction<br />
Bacterial Artificial Chromosomes (BACs) are a very common <strong>and</strong><br />
useful tool for genomic research due to their high cloning efficiency,<br />
clone stability, <strong>and</strong> easy h<strong>and</strong>ling. BACs can maintain DNA<br />
fragments as large as 300 kb while maintaining excellent stability.<br />
Fingerprinting <strong>and</strong> BAC-end sequencing are the major applications<br />
used by scientists to collect genomic sequence data <strong>and</strong> obtaining<br />
quality sequences with long read lengths expedites this research.<br />
It is also advantageous, especially in whole genome sequencing<br />
efforts, to generate sequence data in a high-throughput manner<br />
on fully automated workstations.<br />
The 5 PRIME Perfectprep BAC 96 Kit <strong>and</strong> the <strong>Eppendorf</strong> epMotion<br />
5075 VAC are integrated to provide a complete system for obtaining<br />
highly pure BAC DNA using a fully automated approach. The kit<br />
uses a modified alkaline lysis technology with a vacuum driven<br />
protocol to isolate BAC DNA from bacterial cultures. The protocol<br />
on the epMotion 5075 VAC is optimized to obtain yields of 0.5 –<br />
1.0 μg of highly pure BAC DNA <strong>and</strong> requires approximately<br />
75 minutes. The DNA is ready for immediate use in multiple<br />
downstream applications (at least 5 sequencing reactions, or 4<br />
sequencing reactions <strong>and</strong> 1 fingerprinting analysis, or several <strong>PCR</strong><br />
reactions), <strong>and</strong> the purity of the DNA results in accurate <strong>and</strong> long<br />
sequencing read lengths.<br />
Materials <strong>and</strong> methods<br />
‡ 5 PRIME Perfectprep BAC 96 Kit<br />
‡ <strong>Eppendorf</strong> epMotion 5075 VAC<br />
‡ Human BAC Library plate<br />
Growth of bacterial culture<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Library plate<br />
1.5 ml of 2x YT medium containing 12.5 μg/ml of chloramphenicol<br />
was aliquoted into each well of a 96 deepwell culture plate. Using a<br />
96-pin replicator, the medium was inoculated from frozen glycerol<br />
stocks of one quadrant of a 384-clone BAC library. The cultures<br />
were grown at 37°C overnight for 24 hours in a shaker at 325 rpm.<br />
The plate was centrifuged for 10 minutes at 1900 x g to pellet the<br />
cells, <strong>and</strong> the supernatant was poured off. The plate was blotted to<br />
remove residual medium.<br />
Single clone<br />
15 μl of a single clone BAC gylcerol stock was inoculated into<br />
5 ml 2x YT medium containing 5 μl 12.5 mg/ml chloramphenicol.<br />
This small culture was grown at 37°C at 325 rpm for 6 hours.<br />
600 ml 2x YT medium containing 600 μl 12.5 mg/ml chloramphenicol<br />
was inoculated with 600 μl of the 5 ml starter culture. The<br />
culture was grown overnight (approximately 18 hours) in 37°C<br />
incubator shaking at 325 rpm. 1.5 ml of the culture was aliquoted<br />
into four 96-deep well culture plates so that each well contained<br />
equivalent culture medium. The plates were centrifuged at<br />
1900 x g for 10 minutes. The supernatant was decanted <strong>and</strong><br />
the excess medium was removed by blotting. The pellets were<br />
stored at -20°C until the plates were processed.<br />
Processing<br />
The pelleted cells were processed using the 5 PRIME BAC 96<br />
protocol on the epMotion 5075 VAC. 96 clones from a library plate<br />
were processed. Also, all wells of four plates containing the same<br />
clone (384 samples) were processed.
Isolation of high quality BAC DNA<br />
Isolation of high quality BAC DNA, continued.<br />
Application notes<br />
‡ Figure 1: Screenshot from the epMotion Editor showing the setup of the epMotion 5075 VAC worktable for the 5 PRIME BAC 96 protocol.<br />
Position Labware Comment<br />
A2 epT.I.P.S Motion 1000 μl 96 tips for 96 samples<br />
A3 epT.I.P.S Motion 1000 μl 96 tips for 96 samples<br />
A4 Collection Plate Collects eluates<br />
85 mm Adapter Height adapter for Collection Plate<br />
B1 Position 1: Solution 1<br />
Position 2: Solution 2<br />
Position 3: Solution 3<br />
Position 4: Trapping Buffer<br />
Position 5: Diluted Wash Solution<br />
Position 6: Elution Buffer<br />
30 ml reservoir<br />
30 ml reservoir<br />
30 ml reservoir<br />
30 ml reservoir<br />
100 ml reservoir<br />
30 ml reservoir<br />
B2 epT.I.P.S Motion 1000 μl 24 tips for 96 samples<br />
B3 epT.I.P.S. Motion 300 μl 8 tips for 96 samples<br />
VACUUM Filter Plate A Filters lysate<br />
Vacuum Frame 1 Collar for vacuum chamber<br />
Filter Plate BAC Traps BAC DNA<br />
C1 Culture Plate Contains bacterial pellets<br />
C2 55 mm Adapter Height adapter for Filter Plate BAC<br />
C3 55 mm Adapter Height adapter for Filter Plate A<br />
C4 Vacuum Frame Holder Height adapter for Vacuum Frame 1<br />
T0 Gripper Tool to move labware<br />
T1 TM 1000-8 1000 μl 8-channel pipetting tool<br />
T2 TM 300-8 300 μl 8-channel pipetting tool<br />
‡ Table 1: Details of the worktable used in the ep BAC 96 protocol.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
53<br />
ARTS | Applications
Applications | ARTS<br />
54<br />
Application notes<br />
Isolation of high quality BAC DNA<br />
Isolation of high quality BAC DNA, continued.<br />
Automated fluorescent sequencing<br />
One half of the BAC DNA samples from each plate were sequenced<br />
using ABI BigDye V 3.1 chemistry on an ABI PRISM ® 3700 DNA<br />
Analyzer. The reaction conditions <strong>and</strong> the cycling parameters are<br />
listed in Table 2 <strong>and</strong> Table 3.<br />
Template DNA 10 μl<br />
5x Sequencing Buffer (ABI) 3 μl<br />
BigDye version 3.1 Ready Reaction Premix 2 μl<br />
Primer T7 (10 μM) 1 μl<br />
MgCl 2 (25 mM) 0.6 μl<br />
Molecular Biology Grade H 2 O 3.4 μl<br />
Total Reaction Volume 20 μl<br />
‡ Table 2: Sequencing Reaction Conditions (BigDye version 3.1;<br />
reactions).<br />
Step 1 95°C for 4 minutes<br />
Step 2 95°C for 15 seconds<br />
Step 3 51°C for 15 seconds<br />
Step 4 60°C for 4 minutes<br />
Step 5 Go to step 2 <strong>and</strong> repeat 99 <strong>time</strong>s<br />
Step 6 10°C hold<br />
‡ Table 3: Cycle Sequencing Parameters.<br />
To purify the cycle sequencing products, 5 μl of 125 mM EDTA was<br />
added directly to the samples followed by 60 μl of 95% highly pure<br />
ethanol. The plates were sealed <strong>and</strong> vortexed for 10 seconds. The<br />
samples were incubated for 25 minutes at room temperature in<br />
the dark <strong>and</strong> centrifuged for 30 minutes at 3000 x g. The ethanol<br />
was poured off <strong>and</strong> the plates were spun for 1 minute at 50 x g.<br />
150 μl of ice-cold 70% ethanol was added to wash the samples.<br />
The plates were centrifuged at 1900 x g for 15 minutes. The ethanol<br />
was poured off <strong>and</strong> the plates were spun for 1 minute at 50 x g. The<br />
samples were air dried for at least 15 minutes <strong>and</strong> resuspended in<br />
8 μl of 0.1x TE prior to loading onto the 3700 DNA Analyzer.<br />
Results<br />
Agarose Gel Analysis<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
‡ Figure 2: BAC DNA from one quadrant of a 384-clone library<br />
(96 samples) was isolated using the Perfectprep BAC 96 Kit on<br />
epMotion 5075 VAC. 10 μl of each sample were run on a 1%<br />
agarose gel <strong>and</strong> stained with ethidium bromide. The size marker<br />
is Lambda DNA digested with Hind III.<br />
‡ Figure 3: AC DNA samples from a plate containing the same<br />
single clone in all 96 wells were isolated using the Perfectprep<br />
BAC 96 Kit on the epMotion 5075 VAC. 10 μl of each sample were<br />
analyzed on a 1% agarose gel. Four plates containing this single<br />
clone were processed, <strong>and</strong> this gel photo represents one of those<br />
plates. The size marker is Lambda DNA digested with Hind III.<br />
Library plate<br />
The BAC DNA was sequenced using ABI BigDye V 3.1 chemistry<br />
on an ABI PRISM ® 3700 DNA Analyzer. The average Phred Q>20<br />
score is 676 bases, <strong>and</strong> the passing rate (scores > 100 bases) is<br />
100%. 72% of the scores are over 700 bases.<br />
Single clone<br />
One-half of each of the four plates processed (48 samples for each<br />
plate) was sequenced using ABI BigDye V 3.1 chemistry on an<br />
ABI PRISM ® 3700 DNA Analyzer. The average Phred Q>20 score<br />
for these samples is 718 bases <strong>and</strong> the passing rate (scores > 100<br />
bases) is 97.4%.
Isolation of high quality BAC DNA<br />
Isolation of high quality BAC DNA, continued.<br />
Automated Fluorescent Sequencing<br />
‡ Figure 4: An example of a sequencing trace from this BAC library. The Phred Q>20 score of this<br />
electropherogram is 823 bases, <strong>and</strong> there are zero "N" no calls through 859 bases.<br />
Discussion<br />
BACs continue to be an important molecular biology tool in<br />
genomic research <strong>and</strong> isolation of this DNA is critical to a research<br />
project. Moreover, the yield of BAC DNA must be sufficient <strong>and</strong><br />
the purity must be high for optimal performance in downstream<br />
applications. The Perfectprep BAC 96 Kit provides excellent yield<br />
of high quality BAC DNA in a 96-well format.<br />
This kit is integrated onto the epMotion 5075 VAC system, <strong>and</strong> the<br />
protocol is optimized to give high yields <strong>and</strong> high quality BAC DNA.<br />
Using the Perfectprep BAC 96 Kit, the typical yield of a BAC clone<br />
is 0.5 – 1.0 μg. The average Phred Q>20 sequencing score for the<br />
96 different clones from library plate processed in this paper was<br />
676 bases.<br />
Application notes<br />
This system for purification of BAC DNA also provides consistent<br />
results across a 96-well plate. To demonstrate this, all wells of four<br />
96-well culture plates containing an aliquot of the same flask-grown<br />
single clone were processed with the 5 PRIME kit on the <strong>Eppendorf</strong><br />
system. The agarose gel in Figure 3 illustrates that the yield across<br />
all wells of a plate are consistent. The average Phred Q>20 score<br />
for one half of each of these four plates was 718 with a passing rate<br />
of 100%.<br />
Conclusion<br />
The Perfectprep BAC 96 Kit from 5 PRIME used on the epMotion<br />
5075 VAC from <strong>Eppendorf</strong> is a complete system for the isolation<br />
of BAC DNA in a 96-well, fully automated format. The process<br />
requires approximately 75 minutes <strong>and</strong> gives consistent results<br />
across the plate. The DNA yield is sufficient for several downstream<br />
applications, <strong>and</strong> the DNA purity ensures optimal results from these<br />
applications. High throughput genomic sequencing projects as well<br />
as studies concentrating on specific genes can benefit from this<br />
complete system for the isolation of BAC DNA.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
55<br />
ARTS | Applications
Applications | ARTS<br />
56<br />
Application notes<br />
Accurate <strong>and</strong> precise pipetting of soluents<br />
Accurate <strong>and</strong> precise pipetting of DMSO with the epMotion ® 5070/5075<br />
Daniel Wehrhahn, eppendorf <strong>AG</strong>, Hamburg, Germany<br />
Abstract<br />
Dimethyl sulfoxide (DMSO) is a powerful organic solvent that can<br />
dissolve most organic substances to high loading levels, including<br />
carbohydrates, polymers <strong>and</strong> peptides. Therefore, DMSO is widely<br />
used for compound dissolution in pharmaceutical <strong>and</strong> research<br />
laboratories. Even when performed in the low to mid throughput<br />
range, compound testing assays require a high degree of<br />
st<strong>and</strong>ardization <strong>and</strong> reproducibility. An automated pipetting system<br />
can help to meet these requirements. In this report we show that<br />
DMSO* can be pipetted with the <strong>Eppendorf</strong> epMotion automated<br />
pipetting system at an excellent accuracy <strong>and</strong> precision over a<br />
broad volume range.<br />
Introduction<br />
The excellent pipetting accuracy of the epMotion automated<br />
pipetting system has made it a popular tool for many dem<strong>and</strong>ing<br />
small volume molecular biology reaction set-ups [1]. Routine<br />
pipetting tasks, like serial dilutions, reagent distributions <strong>and</strong><br />
sample transfers can equally benefit from automation through<br />
increased reproducibility <strong>and</strong> complete assay st<strong>and</strong>ardization.<br />
The epMotion pipetting tools work with an air cushion piston<br />
stroke system <strong>and</strong> disposable tips. The unique optical sensor can<br />
measure liquid levels contact free, also with non-conducting liquids<br />
like DMSO. Liquid type parameters for the tools can be adapted to<br />
many different liquids from aqueous solutions to organic solvents.<br />
Safe operation is ensured by the completely contained housing that<br />
prevents manual interference in the process <strong>and</strong> possible contact<br />
with hazardous substances.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
‡ Figure 1: epMotion 5075 LH, Version with PC software.<br />
Materials <strong>and</strong> methods<br />
When working with pure DMSO, the liquid type class “98% alcohol”<br />
should be set in the epMotion software [2]. With this setting a<br />
prewetting step is performed to saturate the air inside the tip with<br />
the solvent <strong>and</strong> the aspiration <strong>and</strong> dispensing is done at low speed.<br />
Water based solutions with low DMSO content should be pipetted<br />
with the liquid type class “water”.<br />
For the accuracy <strong>and</strong> precision measurement of DMSO the<br />
epMotion was equipped with a Mettler S<strong>AG</strong> high precision balance.<br />
The measurements were taken <strong>and</strong> recorded electronically<br />
using the PICASO ® Pipette calibration software [3]. Used tools<br />
were taken r<strong>and</strong>omly from the training laboratory. The density of<br />
DMSO was set to 1.101 mg/μl. For the single channel TS tools<br />
the measurements were taken according to EN ISO 8655: 10<br />
measurements each at 10%, 50% <strong>and</strong> 100% of the maximum<br />
volume of the tool. For each volume, the average out of these 10<br />
measurements, the corresponding systematic error (Accuracy) <strong>and</strong><br />
the r<strong>and</strong>om error (Precision) was calculated.<br />
* Please note that DMSO can be explosive. DMSO should be used<br />
in well ventilated areas when working with concentrated solutions.
Accurate <strong>and</strong> precise pipetting of soluents<br />
Accurate <strong>and</strong> precise pipetting of DMSO, continued.<br />
For the eight channel TM-8 tools 12 measurements in total with<br />
4 separate channels were taken at 10%, 50% <strong>and</strong> 100% of the<br />
maximum filling volume. Per individual channel 3 measurements<br />
were taken. For each volume the average systematic error<br />
(Accuracy) <strong>and</strong> r<strong>and</strong>om error (Precision) were calculated for the<br />
4 channels.<br />
‡ Table 1: Automated pipetting of DMSO with the epMotion system: Accuracy <strong>and</strong> precision results.<br />
Conclusion<br />
The data clearly demonstrate that DMSO can be pipetted by<br />
the epMotion with a very high accuracy <strong>and</strong> precision over<br />
the complete volume range. The results were obtained with<br />
pipetting tools from st<strong>and</strong>ard laboratory equipment <strong>and</strong> were not<br />
precalibrated before the test. This shows the excellent mechanical<br />
quality <strong>and</strong> reproducibility of the tools.<br />
With the single channel pipetting tools a CV below 1% at 5 μl <strong>and</strong><br />
below 0.05% at 1000 μl could be achieved. These values clearly lie<br />
within the <strong>Eppendorf</strong> technical specification limits for the epMotion<br />
tools set for the pipetting of double distilled water.<br />
Application notes<br />
The results are shown in Table 1. The technical specifications<br />
for the epMotion pipetting tools <strong>and</strong> the accuracy <strong>and</strong> precison<br />
limits for the pipetting of double distilled water can be found<br />
in the epMotion manual or on the internet at<br />
www.eppendorf.com/epmotion.<br />
Dispensing tool Volume range Volume Average Systematic error<br />
(Accuracy, es)<br />
R<strong>and</strong>om error<br />
(Precision, CV)<br />
TS 50 1 -50 μl 5 μl 5.21 μl 4.18% 0.84%<br />
25 μl 25.27 μl 1.09% 0.48%<br />
50 μl 50.47 μl 0.95% 0.25%<br />
TS 300 20 - 300 μl 30 μl 30.41 μl 1.36% 0.47%<br />
150 μl 150.1 μl 0.06% 0.13%<br />
300 μl 300.1 μl 0.05% 0.06%<br />
TS 1000 40 - 1000 μl 100 μl 100.97 μl 0.97% 0.36%<br />
500 μl 500.3 μl 0.06% 0.05%<br />
1000 μl 997.0 μl -0.03% 0.02%<br />
TM 50-8 1 -50 μl 5 μl 5.35 μl 6.97% 3.04%<br />
25 μl 25.28 μl 1.13% 0.83%<br />
50 μl 50.37 μl 0.73% 0.40%<br />
TM 300-8 20 - 300 μl 30 μl 30.81 μl 2.70% 0.83%<br />
150 μl 150.98 μl 0.65% 0.19%<br />
300 μl 299.73 μl -0.10% 0.09%<br />
TM 1000-8 40 - 1000 μl 100 μl 101.02 μl 1.02% 0.44%<br />
500 μl 501.4 μl 0.27% 0.03%<br />
1000 μl 1000.13 μl 0.01% 0.01%<br />
References<br />
[1] Accuracy <strong>and</strong> precision of the epMotion system, <strong>Eppendorf</strong> Application Note 104,<br />
December 2004.<br />
[2] epMotion 5070/5075 Operating Manual, <strong>Eppendorf</strong> <strong>AG</strong>, Hamburg.<br />
[3] PICASO Pipette calibration software, <strong>Eppendorf</strong> <strong>AG</strong>, Hamburg.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
57<br />
ARTS | Applications
Applications | ARTS<br />
58<br />
Application notes<br />
Automated high-throughput RNA purification<br />
PureLink 96 Total RNA Purification Kit—automated high-throughput purification of total<br />
RNA from cultured cells<br />
eric Olivares, Invitrogen Corp., Carlsbad, CA; Daniel Wehrhahn, eppendorf <strong>AG</strong>, Hamburg, Germany<br />
Starting material: w 5 × 10 5 cells/well<br />
Typical yields: Up to 25 μg<br />
epMotion ® 5075 VAC processing <strong>time</strong>: 48–53 min<br />
The PureLink 96 Total RNA Purification Kit provides high-<br />
throughput isolation of high-quality total RNA from the widest<br />
range of sample types <strong>and</strong> sizes. The kit allows isolation of high<br />
yields of total RNA with minimal genomic DNA contamination from<br />
bacterial, yeast, plant, or mammalian samples. High-purity total<br />
RNA is eluted into RNase-free water <strong>and</strong> ideal for use in a variety<br />
of applications, including RT-<strong>PCR</strong>, q<strong>PCR</strong>, northern blotting, <strong>and</strong><br />
nuclease protection assays.<br />
Yield (µg)<br />
20<br />
18<br />
16<br />
14<br />
12<br />
10<br />
8<br />
6<br />
4<br />
2<br />
‡ Figure 1: 5 × 10 5 293F cells per well were processed using the<br />
PureLink 96 Total RNA Purification Kit <strong>and</strong> the epMotion ® 5075<br />
VAC platform. Yields were quantitated using the Quant-iT RNA<br />
Assay Kit.<br />
4.7 kb<br />
1.9 kb<br />
4.7 kb<br />
1.9 kb<br />
0<br />
0 10 20 30 40 50 60 70 80<br />
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17<br />
‡ Figure 2: Agarose gel electrophoresis of total RNA isolated with<br />
the PureLink Total RNA Kit from 5 × 10 5 293F cells. Total RNA<br />
(15 μl of eluate, ~9% of total yield) was separated on an E-Gel ® 48<br />
1% agarose gel.<br />
n = 80<br />
Mean yield = 10.1 ± 1.2 µg<br />
CV = 12.5%<br />
Well position<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Cultured 293F cells (5 x 10 5 cells/well) were lysed manually off the<br />
instrument, <strong>and</strong> then the lysates were transferred to a deepwell<br />
plate for automated processing. It is possible to perform the lysis<br />
step directly on the instrument; however, due to the labile nature<br />
of RNA it is preferable to lyse cells immediately after harvesting to<br />
stabilize the cellular transcriptome. The deck setup <strong>and</strong> required<br />
labware <strong>and</strong> accessories are shown in the appendix. Automated<br />
processing using the epMotion ® 5075 VAC system was completed<br />
in just under 45 minutes.<br />
Yields were measured using the Quant-iT RNA Assay Kit<br />
(Figure 1). The total RNA samples were subjected to a variety of<br />
quality measurements. Agarose gel electrophoresis (Figure 2)<br />
<strong>and</strong> Agilent Bioanalyzer analysis (Figure 3) demonstrate that the<br />
isolated RNA is of high integrity <strong>and</strong> contains minimal genomic<br />
DNA contamination. Quantitative RT-<strong>PCR</strong> for a housekeeping gene<br />
was used to assess the performance of the isolated total RNA in<br />
downstream applications. As shown in Figure 4, the RNA produces<br />
linear amplification plots in qRT-<strong>PCR</strong> down to a 105-fold<br />
cDNA dilution.<br />
Bases<br />
4,000<br />
2,000<br />
1,000<br />
500<br />
200<br />
25<br />
1 2 3 4 5 6 7<br />
9.8 9.9 10 10 10 10<br />
RNA integrity number (RIN)<br />
‡ Figure 3: Agilent ® Bioanalyzer analysis of total RNA integrity.<br />
RNA prepared from 5 × 10 5 293F cells using the PureLink<br />
96 Total RNA Kit (1 μl, ~50 ng) was subjected to microcapillary<br />
electrophoresis using an Agilent Bioanalyzer. Control RNA<br />
samples (~250 ng) were also included. The Bioanalyzer calculated<br />
RNA Integrity Numbers (“RIN”) from measurements of the<br />
electrophoretic trace. The PureLink samples all had RINs of 10,<br />
indicating the highest possible integrity. Lane 1: Agilent RNA 6000<br />
Nano LabChip ® Kit size st<strong>and</strong>ard; lanes 2–3: control RNA, 250 μg;<br />
lanes 4–7: four selected RNA samples.
Automated high-throughput RNA purification<br />
Automated high-throughput RNA purification, continued.<br />
A<br />
C t<br />
C t<br />
B<br />
�Rn<br />
45<br />
40<br />
35<br />
30<br />
25<br />
20<br />
45<br />
40<br />
35<br />
30<br />
25<br />
20<br />
1<br />
0.1<br />
10 –5 10 –4 10 –3 10 –2 10 –1 1<br />
cDNA relative dilution<br />
10 –5 10 –4 10 –3 10 –2 10 –1 1<br />
cDNA relative dilution<br />
Ampli�cation plot<br />
C t<br />
C t<br />
0.01<br />
0 5 10 15 20 25<br />
45<br />
40<br />
35<br />
30<br />
25<br />
20<br />
45<br />
40<br />
35<br />
30<br />
25<br />
20<br />
Cycle<br />
10 –5 10 –4 10 –3 10 –2 10 –1 1<br />
cDNA relative dilution<br />
10 –5 10 –4 10 –3 10 –2 10 –1 1<br />
cDNA relative dilution<br />
30 35 40 45 50<br />
Application notes<br />
‡ Figure 4: Isolated total RNA produces linear amplification plots. First-str<strong>and</strong> cDNA was synthesized from ~300 ng of total RNA using<br />
reagents included in the SuperScript ® III First-Str<strong>and</strong> Synthesis SuperMix for qRT-<strong>PCR</strong> kit (Cat. no. 11752-050). A. A ten-fold dilution series<br />
was prepared, <strong>and</strong> 5 μl of each dilution was used in triplicate q<strong>PCR</strong> reactions for β-actin. The certified LUX primer set for ββ-actin (Cat. no.<br />
101H-01) was used in combination with Platinum ® Quantitative <strong>PCR</strong> SuperMix-UDG with ROX (Cat. no. 11743-100). <strong>Real</strong>-<strong>time</strong> detection of<br />
amplification was performed using an ABI 7900HT thermocycler. B. St<strong>and</strong>ard curves were plotted for four r<strong>and</strong>om samples over the 6-log<br />
dilution series, <strong>and</strong> all four samples show a squared correlation coefficient (R 2 ) of 0.997–0.999, demonstrating robust performance of the<br />
isolated RNA in qRT-<strong>PCR</strong>.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
59<br />
ARTS | Applications
Applications | ARTS<br />
60<br />
Application notes<br />
Automated genomic DNA purification<br />
Automated genomic DNA purification in 96-well plate <strong>and</strong> 8-well strip format using the<br />
MACHEREY-N<strong>AG</strong>EL NucleoSpin ® 8/96 Tissue kits on the epMotion ® 5075<br />
Henning Risch, Thomas Zinn, MACHeReY-N<strong>AG</strong>eL GmbH & Co. KG, Düren, Germany; Daniel Wehrhahn, eppendorf <strong>AG</strong>,<br />
Hamburg, Germany<br />
Abstract<br />
In the current application note we demonstrate the integration<br />
of the MACHEREY-N<strong>AG</strong>EL NucleoSpin 8/96 Tissue kits into the<br />
epMotion 5075 VAC automated pipetting system. The NucleoSpin<br />
8/96 Tissue kits are based on a vacuum filtration based bind-washelute<br />
procedure. Protocols for the epMotion 5075 VAC are available<br />
for medium throughput using the flexible 8-well strip based<br />
purification kit or for high throughput using the 96-well plate based<br />
kit. The use of NucleoSpin 8/96 Tissue kits on the epMotion 5075<br />
instrument allows the isolation of DNA from a wide range of sample<br />
materials. Application data for genomic DNA isolation from different<br />
mouse tissues, human cells or bacteria cells are presented.<br />
Introduction<br />
Typically, <strong>PCR</strong> based analytical methods in the field of transgenics,<br />
genotyping <strong>and</strong> SNP analysis require template DNA of high<br />
quality <strong>and</strong> reproducible yields. Purification of genomic DNA from<br />
different types of sample material is still challenging. First, the<br />
method should be sensitive, robust <strong>and</strong> easy to automate with<br />
little h<strong>and</strong>s-on <strong>time</strong>. Second, besides a high degree of flexibility<br />
for the user, the procedure should avoid use of toxic solvents or<br />
precipitations which are very difficult to automate. Most of the<br />
drawbacks of conventional DNA isolation can be overcome by<br />
state-of-the-art silica membrane purification. Here, we describe<br />
the use of the MACHEREY-N<strong>AG</strong>EL NucleoSpin 8/96 Tissue kits for<br />
use on the epMotion 5075 VAC automated pipetting system. Using<br />
the well proven bind-wash-elute procedure liquid-liquid extraction<br />
or DNA precipitation can be avoided. The NucleoSpin 8/96 Tissue<br />
kits provide excellent consistency, a high robustness even when<br />
using very diverse sample types such as tissues from various<br />
organs, mouse tail clippings, eukaryotic <strong>and</strong> bacterial cells, <strong>and</strong><br />
uncompromised DNA yield <strong>and</strong> quality.<br />
In addition, the kits can readily be automated on liquid h<strong>and</strong>ling<br />
systems. The procedure starts with an enzymatic sample digest at<br />
56 °C. This heat incubation step can be either performed externally<br />
or on the instrument which is equipped with a heat incubator. All<br />
further steps are performed at room temperature. Following the<br />
lysis incubation the DNA is bound reversibly to the silica membrane<br />
of the NucleoSpin Tissue Binding Plate or strips. Following washing<br />
steps <strong>and</strong> an ethanol evaporation step the purified DNA is eluted in<br />
water or low salt elution buffer. The purified DNA is suitable for use<br />
in down-stream applications like <strong>PCR</strong>, real-<strong>time</strong> <strong>PCR</strong> or restriction<br />
analysis. The kits are available in either 8-well strip format or<br />
96-well plate format in order to meet the user requirements for<br />
sample throughput. The use of NucleoSpin 8/96 Tissue kits on<br />
the epMotion 5075 automated pipetting system provides excellent<br />
results without the need for extensive programming, optimization<br />
<strong>and</strong> set-up <strong>time</strong>.<br />
Materials <strong>and</strong> methods<br />
‡ <strong>Eppendorf</strong> epMotion 5075 VAC<br />
‡ Vac Thermo Lid (for NucleoSpin 96 Tissue kit only)<br />
‡ Vac Frame 2<br />
‡ Vac Holder<br />
‡ Reservoir 400 ml<br />
‡ Collection Plate Adapter for MN Tube Strips<br />
‡ Channeling Plate<br />
‡ Reservoir Rack with Reagent Reservoirs<br />
‡ MACHEREY-N<strong>AG</strong>EL NucleoSpin 96 Tissue kit<br />
‡ MACHEREY-N<strong>AG</strong>EL NucleoSpin 8 Tissue kit<br />
‡ Tissue samples (e.g. mouse tail clippings, mouse ear punches,<br />
mouse organs), eukaryotic cells (HeLa S3 cells), bacterial cells<br />
Product use limitations <strong>and</strong> safety information<br />
Please read the MACHEREY-N<strong>AG</strong>EL NucleoSpin 8 Tissue or<br />
NucleoSpin 96 Tissue manual before performing the method for<br />
the first <strong>time</strong>.<br />
Determination of yield <strong>and</strong> purity<br />
Yield <strong>and</strong> purity were determined using a microplate reader (Biotek,<br />
Powerwave 200). DNA yield was calculated from A260 values.<br />
Purity was determined by calculating the A260/280 ratio.<br />
Agarose gel electrophoresis<br />
Integrity of DNA <strong>and</strong> results of restriction analysis were analyzed<br />
by TAE agarose gel electrophoresis (1% (w/v) agarose, ethidium<br />
bromide stain).<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Restriction analysis<br />
Approx 1 μg DNA was incubated with EcoRI for 2 h at 37 °C<br />
according to manufacturers instructions (Invitrogen).<br />
‡ Figure 1: Screenshot from the epMotion Editor showing the setup<br />
of the epMotion 5075 VAC worktable for use with the NucleoSpin 96<br />
Tissue kit.
Automated genomic DNA purification<br />
Automated genomic DNA purification, continued.<br />
Position Labware Comment<br />
A2 epT.I.P.S Motion 1000 μl<br />
A3 epT.I.P.S Motion 1000 μl<br />
A4 MN Tube Strips Elution tubes<br />
B1 epT.I.P.S Motion 1000 μl<br />
B2 Reagent Reservoirs<br />
Position 1: Empty<br />
Position 2: Buffer BQ1/Ethanol mix<br />
Position 3: Buffer BW<br />
Position 4: Buffer B5<br />
Position 5: Buffer B5<br />
Position 6: Buffer BE<br />
Position 7: Empty<br />
Optional: Lysis buffer<br />
100 ml reservoir<br />
100 ml reservoir<br />
100 ml reservoir<br />
100 ml reservoir<br />
30 ml reservoir<br />
Empty<br />
B3 1.1 ml deepwell plate Sample plate<br />
Vacuum NucleoSpin Tissue Binding Plate<br />
Vacuum Frame 2<br />
Reservoir 400 ml with channeling plate<br />
DNA binding plate (top)<br />
Collar for vacuum manifold<br />
Collects waste<br />
C3 2.1 ml deepwell plate For mixing sample with binding buffer<br />
C4 Vacuum Frame Holder Height adapter for vacuum Frame 2<br />
T0 Gripper<br />
T1 TM 1000-8 1000 μl 8-channel pipetting tool<br />
‡ Table 1: epMotion 5075 VAC worktable details for NucleoSpin 96 Tissue protocol.<br />
Position Labware Comment<br />
A2 epT.I.P.S Motion 1000 μl<br />
A3 epT.I.P.S Motion 1000 μl<br />
A4 MN Tube Strips Elution tubes<br />
B2 Reagent Reservoirs<br />
Position 1: Empty<br />
Position 2: Buffer BQ1/Ethanol mix<br />
Position 3: Buffer BW<br />
Position 4: Buffer B5<br />
Position 5: Empty<br />
Position 6: Buffer BE<br />
Position 7: Empty<br />
‡ Table 2: epMotion 5075 VAC worktable details for NucleoSpin 8 Tissue protocol.<br />
Optional: Lysis buffer<br />
100 ml reservoir<br />
100 ml reservoir<br />
100 ml reservoir<br />
Empty<br />
30 ml reservoir<br />
Empty<br />
B3 1.1 ml deepwell plate Sample plate<br />
Vacuum NucleoSpin Tissue Binding Strips<br />
Vacuum Frame 2<br />
Reservoir 400 ml with channeling plate<br />
Application notes<br />
DNA binding strips inserted into Column Holder A (top)<br />
Collar for vacuum manifold<br />
Collects waste<br />
C3 2.1 ml deepwell plate For mixing sample with binding buffer<br />
C4 Vacuum Frame Holder Height adapter for vacuum Frame 2<br />
T0 Gripper<br />
T1 TM 1000-8 1000 μl 8-channel pipetting tool<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
61<br />
ARTS | Applications
Applications | ARTS<br />
62<br />
Application notes<br />
Automated genomic DNA purification<br />
Automated genomic DNA purification, continued.<br />
‡ Figure 2: Screenshot from the epMotion Editor showing the<br />
setup of the epMotion 5075 VAC worktable for use with the<br />
NucleoSpin 8 Tissue kit.<br />
DNA yield, µg<br />
DNA purity, A260/280<br />
12<br />
10<br />
8<br />
6<br />
4<br />
2<br />
0<br />
0 8 16 24 32 40 48 56 64 72 80 88 96<br />
2<br />
1<br />
‡ Figure 3: Reproducibility of DNA purification using<br />
NucleoSpin 96 Tissue kit.<br />
Genomic DNA was isolated from mouse tail samples (master<br />
lysate) using NucleoSpin 96 Tissue kit on the epMotion 5075 VAC<br />
automated pipetting system. DNA yield <strong>and</strong> purity were determined<br />
spectrophotometrically.<br />
sample number<br />
0<br />
0 8 16 24 32 40 48 56 64 72 80 88 96<br />
sample number<br />
Results<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Reproducibility of yield <strong>and</strong> purity of genomic DNA isolated<br />
from mouse tail clippings using NucleoSpin 96 Tissue kit<br />
In order to demonstrate the reproducibility of the purification<br />
method genomic DNA was isolated from a master lysate of mouse<br />
tail clippings representing identical sample material. Each lysate<br />
aliquot corresponds to a 4 mm mouse tail sample. The mouse<br />
tail samples were lysed with proteinase K overnight at 56 °C.<br />
Heat incubation for lysis was performed in an external incubator.<br />
Following lysis the lysate was centrifuged in order to precipitate<br />
remaining debris <strong>and</strong> hairs. The cleared lysate was transferred<br />
into the wells of a deepwell plate <strong>and</strong> placed on the instrument for<br />
further processing. DNA yield <strong>and</strong> purity are shown in figure 3. The<br />
results are summarized in table 3. Highly reproducible results for<br />
yield <strong>and</strong> purity were obtained. With a CV of 6.2% an average yield<br />
of 9.3 μg genomic DNA was obtained from the mouse tail clippings.<br />
DNA yield (µg) DNA purity ( A260/280 )<br />
average yield / purity 9.30 1.84<br />
st<strong>and</strong>ard deviation 0.58 0.05<br />
min. yield / purity 8.03 1.68<br />
max. yield / purity 10.69 1.91<br />
‡ Table 3: Yield <strong>and</strong> purity of DNA isolated from mouse tail clippings.<br />
Quality of DNA <strong>and</strong> structural integrity<br />
In order to demonstrate quality <strong>and</strong> structural integrity of the<br />
isolated DNA the purified samples were analyzed by agarose<br />
gel electrophoresis. From the recovered 150 μl of purified DNA<br />
20 μl were loaded on the agarose gel. The DNA migrates as high<br />
molecular weight b<strong>and</strong> of approx. 20-30 kbp. The appearance<br />
of the tight DNA b<strong>and</strong> <strong>and</strong> the absence of low molecular weight<br />
smear demonstrate the excellent quality of DNA <strong>and</strong> well to well<br />
consistency. The results are shown in figure 4.<br />
‡ Figure 4: Agarose gel analysis of purified DNA isolated from<br />
mouse tail clippings.<br />
Aliquots of 20 μl from r<strong>and</strong>omly selected purified genomic DNA<br />
samples were analyzed on an 1% agarose gel. High molecular<br />
weight DNA with a good consistency of yield was obtained.
Automated genomic DNA purification<br />
Automated genomic DNA purification, continued.<br />
Isolation of genomic DNA from different mouse organ tissues<br />
using NucleoSpin 8 Tissue kit<br />
In addition to the 96-well plate based NucleoSpin 96 Tissue kits<br />
for high sample throughput, the NucleoSpin 8 Tissue kit offers<br />
high flexibility using the NucleoSpin 8 Tissue binding strips. This<br />
kit is specially designed for medium throughput <strong>and</strong> allows for the<br />
processing of flexible sample numbers in multiples of 8 samples.<br />
The use of individual 8-well strips avoids the sealing of unused<br />
wells of a 96-well plate when processing less than 96 samples.<br />
Reusing partially used 96-well filterplates introduces a risk of<br />
contamination or sample carry-over <strong>and</strong> thus should be avoided.<br />
As an example for the use of the NucleoSpin 8 Tissue kit DNA<br />
purification from different mouse organs is shown in figure 5. The<br />
results for yield <strong>and</strong> purity are summarized in figure 6. The mouse<br />
organs were incubated for 6 h at 56 °C in the supplied lysis buffer<br />
including proteinase K using an external incubator.<br />
kidney brain liver<br />
heart ear tail<br />
‡ Figure 5: DNA isolation from mouse organs.<br />
DNA was isolated from 20 mg tissue samples as described<br />
before. Aliquots of 20 μl from the purified samples were analyzed<br />
by agarose electrophoresis. High molecular weight DNA with<br />
consistent yield was obtained.<br />
‡ Figure 6: DNA isolation with NucleoSpin 8 Tissue from<br />
different mouse tissue samples.<br />
DNA was isolated from approx. 20 mg of the indicated tissues. Each<br />
bar represents the average of eight extractions. Error bars indicate<br />
the st<strong>and</strong>ard deviation for each set of extractions.<br />
Application notes<br />
DNA yield as shown in figure 6 represents the amount of genomic<br />
DNA in different organs. Different yields are also represented by the<br />
DNA intensities in figure 5. Within one sample (e.g., mouse kidney<br />
tissue) DNA yields are consistent with a small variation as indicated<br />
by the error bars in figure 6.<br />
DNA quality <strong>and</strong> suitability for downstream applications<br />
In order to demonstrate quality (e.g., absence of DNase activity)<br />
<strong>and</strong> suitability for downstream applications, isolated DNA was<br />
analyzed by agarose gel electrophoresis. Samples were analyzed<br />
with <strong>and</strong> without treatment with restriction enzyme. Furthermore,<br />
samples were mixed with restriction enzyme incubation buffer<br />
<strong>and</strong> incubated without restriction enzyme for 2 h at 37 °C to<br />
demonstrate the absence of DNase activity. The results are<br />
shown in figure 7.<br />
‡ Figure 7: Restriction digest of isolated genomic DNA.<br />
An aliquot of 10 μl from r<strong>and</strong>omly selected purified DNA samples<br />
isolated from mouse tail clippings were incubated for 2 h at 37 °C<br />
with EcoRI restriction enzyme (+). In all samples treated with EcoRI<br />
enzyme DNA was restricted. Another aliquot was incubated with<br />
the restriction enzyme buffer only at 37 °C for 2 h (-). The samples<br />
incubated with enzyme reaction buffer only show a distinct high<br />
molecular weight b<strong>and</strong>. Distinct b<strong>and</strong>s in these samples indicate<br />
the absence of DNase activity demonstrating the high quality of<br />
DNA.<br />
In order to demonstrate the suitability of the purified DNA for<br />
real-<strong>time</strong> <strong>PCR</strong> analysis, DNA isolated from mouse tail samples<br />
was used as template for <strong>PCR</strong> in a Roche ® Lightcycler instrument.<br />
The purified DNA was diluted 1:10 <strong>and</strong> used in 40 cycle reactions.<br />
A primer set amplifying a 212 bp fragment of Mus musculus<br />
cytoplasmatic aconitase exon (aco I) gene was used.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
63<br />
ARTS | Applications
Applications | ARTS<br />
64<br />
Application notes<br />
Automated genomic DNA purification<br />
Automated genomic DNA purification, continued.<br />
Figure 8: <strong>Real</strong> <strong>time</strong> <strong>PCR</strong> of DNA isolated from mouse tails.<br />
DNA isolated from mouse tails was used in a SYBR Green <strong>PCR</strong><br />
assay. <strong>PCR</strong> amplification plots show reproducible amplification <strong>and</strong><br />
no evidence for <strong>PCR</strong> inhibitors. The average crossing point for all<br />
sixteen samples was 23.79 with a CV of 2.43% demonstrating the<br />
high reproducibility of DNA yield <strong>and</strong> amplification. The specificity<br />
of the amplified <strong>PCR</strong> product was verified by melting curve<br />
analysis <strong>and</strong> analysis of the amplified <strong>PCR</strong> products by agarose<br />
electrophoresis (data not shown).<br />
DNA isolation from cells<br />
With a slightly modified procedure the NucleoSpin 8/96 Tissue<br />
kits can also be used for DNA isolation from cultured eukaryotic or<br />
bacterial cells. DNA isolation from eukaryotic cells was exemplified<br />
with HeLa cells. In contrast to the applications described before<br />
the lysis with proteinase K supplemented buffer <strong>and</strong> all purification<br />
steps were performed at room temperature on the instrument.<br />
After lysis RNase A was added to digest RNA. The results of the<br />
extraction of gDNA from HeLa cells are shown in the figure 9.<br />
‡ Figure 9: DNA isolation from HeLa cells<br />
HeLa cells were grown in culture bottles, harvested after trypsin<br />
treatment <strong>and</strong> pelleted into the wells of the lysis block. Each well<br />
represents approx. 5 x 10 5 cells. gDNA was isolated according to<br />
the NucleoSpin 96 Tissue protocol using lysis with proteinase K<br />
followed by RNase A treatment. Average yield of DNA was 2.9 μg.<br />
The high molecular weight b<strong>and</strong> <strong>and</strong> absence of smear indicate the<br />
excellent quality of the DNA.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
DNA isolation from bacteria cells<br />
DNA isolation from gram-negative bacteria may be performed<br />
according to the st<strong>and</strong>ard protocol of the NucleoSpin 8/96 Tissue<br />
kit protocol. For Gram-positive bacteria the lysis procedure has to<br />
be modified. For these difficult to lyse bacteria the use of a modified<br />
lysis buffer, including lysozyme or lysostaphin, is recommended.<br />
M ------B. subtilis----- ----B. polymyxia----<br />
‡ Figure 10: DNA isolation from bacterial cells<br />
DNA was isolated from 1 ml of an overnight culture of Bacillus<br />
subtilis <strong>and</strong> Bacillus polymyxa. Bacteria cells were harvested <strong>and</strong><br />
resuspended in 180 μl resuspension buffer (20 mM Tris buffer pH<br />
8,0, 2 mM EDTA, 1% Triton X-100). 25 μl of a lysozyme solution<br />
(20 mg/ml) was added to each sample. Bacteria were lysed for<br />
1 h at 37°C with moderate shaking. Following lysis 20 μl of a RNase<br />
A solution (20 mg/ml) was added <strong>and</strong> samples were incubated<br />
for 10 min at room temperature. All further steps were performed<br />
according to the st<strong>and</strong>ard protocol of NucleoSpin ® 96 Tissue kit.<br />
Reproducible yields of high molecular weight DNA were obtained.<br />
Conclusion<br />
The combination of the MACHEREY-N<strong>AG</strong>EL NucleoSpin 8 Tissue<br />
<strong>and</strong> 96 Tissue kits <strong>and</strong> the epMotion 5075 VAC resulted in a flexible<br />
system for automated purification of high quality genomic DNA<br />
from a broad range of various sample materials. The compact<br />
epMotion 5075 VAC automated pipetting system can be used<br />
either for low to medium throughput using the 8-well strip based<br />
NucleoSpin 8 Tissue kit or for higher throughput using the 96well<br />
based NucleoSpin 96 Tissue kit. Both kits can be used with<br />
the same hardware allowing the user to switch between the two<br />
methods according to the requirements in sample throughput.<br />
DNA purification is achieved using the NucleoSpin Technology<br />
based on a vacuum driven bindwash-elute procedure. The use<br />
of an optimized silica membrane in the NucleoSpin 8/96 Tissue<br />
kits allows the purification of DNA from various sample materials<br />
without a risk of clogged columns. The purified DNA is of excellent<br />
quality <strong>and</strong> suitable for downstream applications such as restriction<br />
analysis or <strong>PCR</strong> based analysis. In summary, the NucleoSpin<br />
technology <strong>and</strong> the epMotion 5075 VAC automated pipetting<br />
system form an attractive <strong>and</strong> versatile system for the automated<br />
isolation of genomic DNA from different sample materials.
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> with Mastercycler® ep realplex<br />
Mastercycler ep realplex—a flexible device for fast <strong>and</strong> accurate real-<strong>time</strong> <strong>PCR</strong><br />
Beate Riekens <strong>and</strong> Andrés Jarrin, eppendorf <strong>AG</strong>, Hamburg; Richard Black, eppendorf North America<br />
Introduction<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> has become one of the most popular tools in<br />
molecular biology research. It allows precise quantitative <strong>and</strong><br />
qualitative detection of nucleic acids, <strong>and</strong> it is used in a variety<br />
of applications such as gene expression analysis <strong>and</strong> genotyping.<br />
In addition to fluorochrome-coupled hybridization probes,<br />
intercalating dyes are used for the monitoring of DNA amplification.<br />
Newer developments in real-<strong>time</strong> <strong>PCR</strong> devices <strong>and</strong> reagent<br />
technology concentrate primarily on shortening run <strong>time</strong>s <strong>and</strong><br />
improving sensitivity <strong>and</strong> reproducibility. With Mastercycler ep<br />
realplex, <strong>Eppendorf</strong> introduces a flexible, reliable <strong>and</strong> exceptionally<br />
high-speed real-<strong>time</strong> <strong>PCR</strong> instrument. The device is of modular<br />
design—consisting of a compact thermoblock <strong>and</strong> an optical<br />
detection unit that features an LED optics array <strong>and</strong> the latest<br />
in photo-multiplier technology. The realplex 2 module detects<br />
fluorochromes within a range of 520 to 550 nm, while the realplex 4<br />
device has additional filters, paired with a second channel photo-<br />
multiplier, to detect additional emission wavelengths of 580 to<br />
605 nm. The signal recording <strong>time</strong>s for all 96 samples range from<br />
8 seconds for two channels to a maximum of 16 seconds for all<br />
four detection channels. The optical units can be combined with<br />
either a st<strong>and</strong>ard 96-well aluminum thermoblock or the highspeed<br />
(6 °C/s) 96-well silver block. Both thermoblocks1 have a<br />
temperature gradient option for easier <strong>and</strong> faster optimization of<br />
assays. Depending upon application requirements, Mastercycler ep<br />
realplex can thus be configured to function as a st<strong>and</strong>ard device for<br />
two-fold multiplexing, or, in its most advanced configuration, as a<br />
“high-speed” system for fast real-<strong>time</strong> <strong>PCR</strong> analyses with up to four<br />
detection channels.<br />
In addition to the technical features of the instrument, reagent<br />
chemistry significantly impacts the results <strong>and</strong> protocols of a<br />
real-<strong>time</strong> <strong>PCR</strong> assay. 5 PRIME <strong>Real</strong>MasterMix series reagents<br />
are based on the innovative HotMaster ® technology2 —they<br />
utilize a temperature-dependent inhibitory lig<strong>and</strong> of Taq DNA<br />
polymerase to ensure the Hot Start function. While Taq activity<br />
is blocked at lower temperatures, enzyme activity is immediately<br />
restored when a temperature of 60 °C is exceeded. Therefore, in<br />
contrast to conventional real-<strong>time</strong> <strong>PCR</strong> reagents, <strong>Real</strong>MasterMix<br />
Probe (for use with probe-based assays) <strong>and</strong> <strong>Real</strong>MasterMix (for<br />
SYBR ® Green assays <strong>and</strong> single-plex probe formats) do not require<br />
traditional, lengthy activation steps. Regardless of the reagent<br />
chemistries used, Mastercycler ep realplex’s combined benefit of<br />
high-temperature control speed <strong>and</strong> brief signal recording <strong>time</strong><br />
enables total run <strong>time</strong>s that were never before possible with Peltier<br />
element-based real-<strong>time</strong> <strong>PCR</strong> devices. The performance of<br />
Mastercycler ep realplex, using <strong>Real</strong>MasterMix reagents, is<br />
documented in this article. Experimental data is presented to<br />
highlight reproducibility, linear detection range, sensitivity,<br />
specificity, multiplexing capability <strong>and</strong> speed.<br />
Application notes<br />
Materials <strong>and</strong> methods<br />
All experiments were conducted on a Mastercycler ep<br />
realplex4 S with a silver block. For SYBR Green I assays, 5 PRIME<br />
<strong>Real</strong>MasterMix was used; for TaqMan ® assays, <strong>Real</strong>MasterMix<br />
Probe was used. All reactions were set up on an <strong>Eppendorf</strong><br />
epMotion ® 5070 automated pipetting system <strong>and</strong> dispensed into<br />
<strong>Eppendorf</strong> ® twin.tec 96 skirted <strong>PCR</strong> plates. The plates were then<br />
sealed with <strong>Eppendorf</strong> Heat Sealing Film, using an <strong>Eppendorf</strong><br />
Heat Sealer.<br />
Reproducibility<br />
An 80 bp target sequence of the human sex-related Y chromosome<br />
gene (SRY) was quantitated in a uniformity experiment to determine<br />
the reproducibility of a SYBR Green I assay across all positions of<br />
the thermoblock. 100,000 copies of a plasmid target that contains<br />
the SRY amplicon were used in 50 μl total reaction volumes.<br />
A mastermix of all reaction components was prepared <strong>and</strong><br />
distributed with the 8-channel dispensing tool of the epMotion<br />
5070 into a twin.tec 96 <strong>PCR</strong> plate. The <strong>PCR</strong> run profile A was<br />
used (see Table 2 on page 68).<br />
Following the <strong>PCR</strong>, a melting curve analysis was performed to test<br />
the specificity of the amplification.<br />
1 U.S. Pat. 6,767,512<br />
2 U.S. Pat. 6,667,165<br />
‡ Fig. 1: Mastercycler ep realplex<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
65<br />
ARTS | Applications
Applications | ARTS<br />
66<br />
Application notes<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> with Mastercycler® ep realplex<br />
Mastercycler ep realplex—a flexible device for fast <strong>and</strong> accurate real-<strong>time</strong> <strong>PCR</strong>, continued.<br />
Target sequence Primer/probe sequence TM (°C) Final concentration Amplicon size<br />
Lambda genome Forward primer: caggaactgaagaatgccagaga 63.0 TaqMan ® : 300 nM 104 bp<br />
Reverse primer: ccgtcgagaatactggcaattt 62.5<br />
Probes:<br />
FAM-tgtactttcgtgctgtcgcggatcg-TAMRA<br />
YY-tgtactttcgtgctgtcgcggatcg-BHQ1<br />
72.8 TaqMan single-plex: 200 nM<br />
Sex-related<br />
Forward primer: gcgacccatgaacgcatt 63.0 SYBR<br />
Y chromosome<br />
(SRY) gene<br />
® Green: 300 nM 80 bp<br />
Reverse primer: agtttcgcattctgggattctct 62.6<br />
TaqMan single-plex: 900 nM<br />
TaqMan duplex: 300 nM<br />
Probes:<br />
72.4 TaqMan single-plex: 300 nM<br />
FAM-tggtctcgcgatcagaggcgc-TAMRA<br />
YY-tggtctcgcgatcagaggcgc-BHQ1<br />
TaqMan duplex: 200 nM<br />
GAPDH gene Forward primer: tgccttcttgcctcttgtct 60.1 TaqMan duplex: 300 nM 146 bp<br />
Reverse primer: ggctcaccatgtagcactca 59.9<br />
Probe: FAM-tttggtcgtattgggcgcctgg-BHQ1 71.8 TaqMan duplex: 200 nM<br />
‡ Table 1: Primer <strong>and</strong> probes<br />
Dynamic detection range<br />
With the help of the epMotion ® 5070, a Lambda DNA dilution series<br />
of 10 8 to 10 0 copies/μl was produced. 10 μl of each dilution was<br />
used as template DNA in a Lambda-specific TaqMan assay (see<br />
Table 1). To test comparability of the results of two detection<br />
channels, a FAM- <strong>and</strong> a Yakima Yellow (YY)-labeled probe of the<br />
same sequence were used in parallel experiments. The run profile<br />
B was used (see Table 2 on page 68).<br />
A B<br />
‡ Fig. 2: Reproducibility across 96 replicates<br />
(A): Logarithmically scaled amplification plots of 96 replicates of the SRY target amplified using 5 PRIME <strong>Real</strong>MasterMix in the SYBR<br />
format; with a mean C t value of 18.99, the st<strong>and</strong>ard deviation was 0.07. (B): Melting curve analysis of the 96 replicates shows no primer-<br />
dimers or nonspecific products. Comparable results were obtained with 20 μl of reaction volumes (not shown).<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Differentiation of two-fold concentration differences<br />
A TaqMan assay for the human SRY gene was selected to detect<br />
two-fold concentration differences of template DNA copy numbers.<br />
Starting from a stock solution of human genomic DNA (200 ng/μl,<br />
corresponding to 33,333 copies/μl), samples of 2,000, 1,000, 500<br />
<strong>and</strong> 250 copies, respectively, were produced. For each dilution a<br />
mastermix with 6 replicates was prepared. The run profile B was<br />
used (see Table 2 on page 68).
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> with Mastercycler® ep realplex<br />
Mastercycler ep realplex—a flexible device for fast <strong>and</strong> accurate real-<strong>time</strong> <strong>PCR</strong>, continued.<br />
A B<br />
‡ Fig. 3: Dynamic range in the 520 nm <strong>and</strong> 550 nm detection channel<br />
Application notes<br />
Within a range from 10 9 to 10 1 template molecules, Lambda DNA can be reliably detected in a TaqMan ® assay. Using <strong>Real</strong>MasterMix Probe,<br />
both a FAM-labeled probe [detection channel 520 nm (A)] <strong>and</strong> a Yakima Yellow-labeled probe [detection channel 550 nm (B)] demonstrate<br />
<strong>PCR</strong> efficiencies of 97% <strong>and</strong> 96% at a correlation coefficient of 0.999.<br />
‡ Fig. 4: Two-fold concentration changes<br />
A SRY-specific TaqMan assay shows that Mastercycler ep<br />
realplex can differentiate two-fold copy number differences with<br />
high precision. The C t differences between two-fold differential<br />
dilutions in a range between 2,000 <strong>and</strong> 250 copies correspond<br />
almost exactly to one cycle that mirrors the two-fold differences<br />
on copy numbers.<br />
‡ Fig. 5: Single-molecule DNA detection<br />
Out of 50 replicates of the SRY-specific TaqMan assay,<br />
each statistically calculated to contain a single template DNA<br />
molecule, 28 exceeded threshold while 22 behaved in accordance<br />
with the negative controls. The positive samples, with a mean value<br />
of 37.03, had minimum <strong>and</strong> maximum values of 35.89 <strong>and</strong> 38.07,<br />
respectively. Mastercycler ep realplex in combination with<br />
<strong>Real</strong>MasterMix Probe thus reliably detected single-target<br />
DNA molecules.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
67<br />
ARTS | Applications
Applications | ARTS<br />
68<br />
Application notes<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> with Mastercycler® ep realplex<br />
Mastercycler ep realplex—a flexible device for fast <strong>and</strong> accurate real-<strong>time</strong> <strong>PCR</strong>, continued.<br />
St<strong>and</strong>ard <strong>PCR</strong> profile<br />
Two-step <strong>PCR</strong><br />
Conventional q<strong>PCR</strong> instrument<br />
+ conventional reagents<br />
50 μl reaction volume<br />
<strong>PCR</strong> profile A<br />
Two-step <strong>PCR</strong><br />
Mastercycler ep realplex S +<br />
<strong>Real</strong>MasterMix Probe<br />
Tube control<br />
50 μl reaction volume<br />
<strong>PCR</strong> profile B<br />
Fast two-step <strong>PCR</strong><br />
Mastercycler ep realplex S +<br />
<strong>Real</strong>MasterMix Probe<br />
Tube control<br />
20 μl reaction volume<br />
<strong>PCR</strong> profile C<br />
Fast two-step <strong>PCR</strong><br />
Mastercycler ep realplex S +<br />
<strong>Real</strong>MasterMix Probe<br />
Tube control<br />
20 μl reaction volume<br />
<strong>PCR</strong> profile D<br />
Fast two-step <strong>PCR</strong><br />
Mastercycler ep realplex S +<br />
<strong>Real</strong>MasterMix Probe<br />
Block control<br />
10 μl reaction volume<br />
‡ Table 2: <strong>PCR</strong> profiles<br />
Single-molecule DNA detection<br />
Initial<br />
denaturation/<br />
activation<br />
The SRY gene is present as a single copy on the male Y chromo-<br />
some. A dilution of male human genomic DNA containing 600 fg<br />
DNA/μl was prepared, which corresponds to one copy per 10 μl.<br />
A SRY-specific TaqMan ® assay was developed with the goal of<br />
single-copy detection. As a result of the statistical dispersal of<br />
molecules per volume unit, some reactions contained several<br />
copies each while others contained no target molecules. A<br />
mastermix of 50 replicates, each with 10 μl of template DNA per<br />
reaction, was prepared, <strong>and</strong> run profile B was used (see Table 2).<br />
Denaturation Annealing/<br />
extension<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Cycles Total run <strong>time</strong><br />
10 min 15 s 60 s 40 1 h 38 min<br />
2 min 15 s 60 s 40 1 h 12 min<br />
2 min 10 s 20 s 40 42 min 02 s<br />
20 s 3 s 20 s 40 35 min 37 s<br />
20 s 3 s 17 s 40 23 min 34 s<br />
High-speed <strong>PCR</strong> protocols<br />
The SRY TaqMan assay was selected to test the influence of <strong>PCR</strong><br />
run profiles <strong>and</strong> reaction volumes on the results. A mastermix with<br />
1,000 copies of human gDNA/μl was used (for run profiles see<br />
Table 2). At least 15 replicates were prepared. An identical assay<br />
that uses a traditional two-step <strong>PCR</strong> profile was run in parallel on a<br />
competitor instrument (data not shown).
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> with Mastercycler® ep realplex<br />
Mastercycler ep realplex—a flexible device for fast <strong>and</strong> accurate real-<strong>time</strong> <strong>PCR</strong>, continued.<br />
A B<br />
Av.Ct 22.62<br />
St.dev. 0.05<br />
1 h 12 min<br />
50 μl<br />
C D<br />
Av.Ct 22.45<br />
St.dev. 0.10<br />
32 min<br />
20 μl<br />
‡ Fig 6: High-speed <strong>PCR</strong> profiles<br />
Application notes<br />
(A) to (D) show the effect of a stepwise <strong>time</strong> optimization of <strong>PCR</strong> profiles <strong>and</strong> concomitant reduction of reaction volumes on total <strong>PCR</strong><br />
run <strong>time</strong>s as well as st<strong>and</strong>ard deviation of a SRY TaqMan ® assay. Please note that due to the different reaction volumes <strong>and</strong> fluorescent<br />
intensities generated, C t values cannot be directly compared.<br />
Multiplexing<br />
The human SRY (single copy) <strong>and</strong> GAPDH (two copies per genome)<br />
genes were chosen to demonstrate the multiplexing capability of<br />
Mastercycler ep realplex. In a duplex TaqMan assay a YY/BHQ1-<br />
labeled TaqMan probe for the SRY gene <strong>and</strong> a FAM/BHQ1-labeled<br />
probe for GAPDH gene were used. 10,000 copies of male genomic<br />
DNA per 20 μl reaction were used as template DNA. For primer<br />
<strong>and</strong> probe concentrations see Table 1 on page 68; the run profile<br />
B was used (see Table 2 on the previous page).<br />
A comparison of the <strong>Eppendorf</strong> real-<strong>time</strong> <strong>PCR</strong> system<br />
(Mastercycler ep realplex <strong>and</strong> 5 PRIME <strong>Real</strong>MasterMix reagents)<br />
with a competitor-based system that uses a conventional device<br />
<strong>and</strong> reagent technology shows that considerably shorter run<br />
<strong>time</strong>s can be obtained with the <strong>Eppendorf</strong> system—even when<br />
traditional <strong>PCR</strong> profiles are maintained (Table 2 <strong>and</strong> Fig. 6A).<br />
Av.Ct 22.82<br />
St.dev. 0.08<br />
42 min<br />
20 μl<br />
Av.Ct 23.54<br />
St.dev. 0.08<br />
23 min<br />
10 μl<br />
When the <strong>PCR</strong> profiles are more closely adapted to the properties<br />
of the <strong>Eppendorf</strong> system (fast heating/cooling rates, brief signal<br />
recording <strong>time</strong>s, no enzyme activation <strong>time</strong>s), a run <strong>time</strong> of less<br />
than 45 minutes for 40 cycles can be achieved (Fig. 6B). When<br />
further optimization of the temperature holding <strong>time</strong>s is carried<br />
out, run <strong>time</strong>s of slightly more than 35 minutes with comparable<br />
C values <strong>and</strong> deviations are possible (Fig. 6C).<br />
t<br />
Combined with a reduced reaction volume (5 μl is achievable<br />
with an automated pipetting system), real-<strong>time</strong> <strong>PCR</strong> assays in<br />
the 96-well format with total run <strong>time</strong>s under 24 minutes can be<br />
achieved (Fig. 6D). If established <strong>PCR</strong> systems require slow heating<br />
<strong>and</strong> cooling rates, the speed of Mastercycler ep realplex can be<br />
finely regulated as well as emulate the temperature control behavior<br />
of other devices.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
69<br />
ARTS | Applications
Applications | ARTS<br />
70<br />
Application notes<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> with Mastercycler® ep realplex<br />
Mastercycler ep realplex—a flexible device for fast <strong>and</strong> accurate real-<strong>time</strong> <strong>PCR</strong>, continued.<br />
‡ Fig. 7: Multiplexing of SRY <strong>and</strong> GAPDH targets<br />
A duplex TaqMan ® assay for the SRY (single copy, YY Probe) <strong>and</strong> GAPDH (two copies, FAM probe) targets from human genomic DNA in<br />
12 replicates is shown. As seen in Fig. 3, the YY signal was detected in the 550 nm channel using VIC dye calibration data. The “all dyes”<br />
view of the realplex software reveals the shifted pattern of the GAPDH <strong>and</strong> SRY amplification plots <strong>and</strong> corresponding C t values. Reflecting<br />
the gene dosage ratios of both genomic targets, these data document that Mastercycler ep realplex can reliably differentiate fluorescence<br />
signals in multiplex applications, even with targets that show only minimal differences in abundance.<br />
Conclusion<br />
Mastercycler ep realplex is a real-<strong>time</strong> <strong>PCR</strong> instrument that, due<br />
to its flexible modular design, can be adjusted to the specific<br />
requirements of the user. Its high-quality construction <strong>and</strong> stable,<br />
user-friendly software form a reliable real-<strong>time</strong> <strong>PCR</strong> platform<br />
that ensures a high degree of accuracy across all sample<br />
positions—<strong>and</strong> within a wide detection range. Adoption of the silver<br />
block significantly speeds up run <strong>time</strong>s while still maintaining the<br />
instrument’s high degree of accuracy.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.
Low volume real-<strong>time</strong> <strong>PCR</strong><br />
Low volume real-<strong>time</strong> <strong>PCR</strong> on the Mastercycler ® ep realplex<br />
Nils Gerke, eppendorf <strong>AG</strong>, Hamburg, Germany<br />
Abstract<br />
This Application Note provides a comparative overview of real-<strong>time</strong><br />
<strong>PCR</strong> reactions at 20 µl, 10 µl <strong>and</strong> 5 µl reaction volumes, each<br />
generated on the Mastercycler ep realplex real-<strong>time</strong> <strong>PCR</strong> system.<br />
SYBR ® Green I- as well as TaqMan® probe-based assays were<br />
performed, <strong>and</strong> the reaction setup was done with the aid of the<br />
epMotion ® 5070 automated pipetting system.<br />
It is shown that real-<strong>time</strong> <strong>PCR</strong> reactions with small reaction volumes<br />
can successfully be performed on the Mastercycler ep realplex: The<br />
reaction efficiencies obtained for the presented assays, including<br />
the lowest volume reactions, were comparably good <strong>and</strong> the C t<br />
values obtained from the 10 µl- <strong>and</strong> 5 µl- reactions were only<br />
slightly higher than those detected for the 20 µl setups.<br />
Introduction<br />
While <strong>PCR</strong> setups of 50 μl or 25 μl used to be customary, there<br />
is nowadays a trend towards carrying out <strong>PCR</strong> in lower reaction<br />
volumes [1].<br />
One of the advantages when working with reduced volumes is that<br />
a correspondingly lower amount of template is needed to achieve<br />
a particular DNA template concentration in the reaction sample<br />
(Table 1). This is of particular importance when only small amounts<br />
of template are available, for example in gene expression studies<br />
using degraded biological starting materials or when working with<br />
forensic sample material [1].<br />
As an additional benefit, faster run <strong>time</strong>s can be achieved by<br />
reducing the temperature holding <strong>time</strong>s in the <strong>PCR</strong> protocol, since<br />
the programmed temperatures are transferred to the sample more<br />
quickly in a smaller reaction volume.<br />
However, in many cases the main reason for reducing the reaction<br />
volume is to minimize reagent costs. These reagents, in particular<br />
the kits <strong>and</strong> the fluorescence-labeled DNA probes, comprise a<br />
significant share of total cost during routine use of real-<strong>time</strong> <strong>PCR</strong>.<br />
Therefore, depending on the sample throughput of the laboratory,<br />
significant savings can be achieved by reducing the reaction<br />
volume.<br />
Application notes<br />
Materials <strong>and</strong> methods<br />
With real-<strong>time</strong> <strong>PCR</strong>, two different targets were amplified:<br />
1 <strong>PCR</strong> target: 108 bp, fragment of Lambda DNA<br />
Forward primer (600 nM): cgcacaggaactgaagaatg,<br />
Reverse primer (300 nM): ccgtcgagaatactggcaat,<br />
Lambda TaqMan probe (labeled with FAM, 200 nM):<br />
tgtactttcgtgctgtcgcggatcg<br />
Template: Lambda DNA (Roche)<br />
2 <strong>PCR</strong> target: 80 bp, fragment of human SRY gene<br />
Forward primer (300 nM): gcgacccatgaacgcatt,<br />
Reverse primer (300 nM): agtttcgcattctgggattctct,<br />
TaqMan probe (labeled with FAM, 200 nM): tggtctcgcgatcagaggcgc<br />
Template: Human Genomic DNA (Roche)<br />
For each <strong>PCR</strong> reaction SYBR Green I- <strong>and</strong> TaqMan probe-based<br />
assays were carried out <strong>and</strong> each setup was done with<br />
20 μl, 10 μl, <strong>and</strong> 5 μl reaction volumes. For pipetting the reaction<br />
setup, a Mastermix (consisting of 5 PRIME <strong>Real</strong>MasterMix, SYBR<br />
Green <strong>and</strong> primer or 5 PRIME <strong>Real</strong>MasterMix Probe, TaqMan probe<br />
<strong>and</strong> primer) <strong>and</strong> the highest template DNA concentration were each<br />
initially prepared manually for each reaction (Table 1). In subsequent<br />
steps, the reaction samples were pipetted with the epMotion 5070<br />
automated pipetting system.<br />
The epMotion was programmed to dispense Mastermix into the<br />
corresponding wells of an <strong>Eppendorf</strong> twin.tec <strong>PCR</strong> Plate 96 skirted,<br />
to pipette a dilution series starting with the highest template<br />
concentration <strong>and</strong> then to add template DNA to the appropriate<br />
wells:<br />
20 μl total reaction volume: 12 μl Mastermix + 8 μl template DNA<br />
10 μl total reaction volume: 6 μl Mastermix + 4 μl template DNA<br />
5 μl total reaction volume: 3 μl Mastermix + 2 μl template DNA<br />
A 5-step serial dilution with five replicates each was pipetted to<br />
generate a st<strong>and</strong>ard curve that permitted comparison of the <strong>PCR</strong><br />
results. While the Lambda DNA st<strong>and</strong>ard samples were diluted<br />
ten-fold, the st<strong>and</strong>ard samples for human genomic DNA were<br />
diluted three-fold. A negative control was included in each assay<br />
by substituting water for template DNA.<br />
The <strong>Eppendorf</strong> twin.tec <strong>PCR</strong> Plates 96 skirted were sealed with<br />
<strong>Eppendorf</strong> Heat Sealing Film <strong>and</strong> then subjected to a short spin<br />
(approx. 500 x g) prior to the run. The <strong>PCR</strong> setup for all assays<br />
was carried out with the same epMotion 5070 automated pipetting<br />
system <strong>and</strong> all real-<strong>time</strong> <strong>PCR</strong> runs were performed on the same<br />
Mastercycler ep realplex4 S device (Table 2).<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
71<br />
ARTS | Applications
Applications | ARTS<br />
72<br />
Application notes<br />
Low volume real-<strong>time</strong> <strong>PCR</strong><br />
Low volume real-<strong>time</strong> <strong>PCR</strong> on the Mastercycler ® ep realplex, continued.<br />
‡ Figure 1: Selected amplification plots <strong>and</strong> parameters of the 20 μl, 10 μl und 5 μl Lambda (A, B) <strong>and</strong> SRY (C, D) assays.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.
Low volume real-<strong>time</strong> <strong>PCR</strong><br />
Low volume real-<strong>time</strong> <strong>PCR</strong> on the Mastercycler ® ep realplex, continued.<br />
Results<br />
The amplification plots of the presented assays are comparably<br />
steep for all reaction volumes (Figure 1). Absolute fluorescence<br />
intensity is lower at the smaller reaction volumes. However, even in<br />
the case of the lowest fluorescence intensity (5 μl SRY SYBR assay)<br />
the analysis can be performed easily with adjusted scaling.<br />
<strong>PCR</strong> efficiencies of the shown assays, calculated from the C values<br />
t<br />
of the st<strong>and</strong>ard samples, differ only minimally among the various<br />
reaction volumes of the particular <strong>PCR</strong> system. The r2-coefficients of the Lambda assays do not show a decline at smaller reaction<br />
volumes (≥ 0.99 for all). The r2- values of the 5 μl setups for the SRY<br />
assays are, with values of 0.989 <strong>and</strong> 0.987, slightly below the 10 μl<br />
<strong>and</strong> 20 μl setups (Figure 1).<br />
When considering all assays shown in Figure 1, the mean replicate<br />
C values of the 10 μl reaction samples are, at the highest template<br />
t<br />
concentration level, between 0.55 <strong>and</strong> 0.86 higher compared to the<br />
20 μl reactions. For the 5 μl setups an increase of between 0.27 <strong>and</strong><br />
0.61 relative to the 10 μl assays was observed.<br />
Reaction<br />
Setup<br />
highest<br />
template<br />
conc.<br />
[cop./μl]<br />
Lambda SRY<br />
template<br />
amount<br />
[cop./rxn]<br />
highest<br />
template<br />
conc.<br />
[ng/μl]<br />
template<br />
amount<br />
[ng/rxn]<br />
20 μl<br />
2.5x105 5x106 24.3<br />
10 μl 2.5x10 1.215<br />
6 12.15<br />
5 μl 1.25x106 6.075<br />
‡ Table 1: Highest template concentration <strong>and</strong> its appropriate<br />
template amount for the 20 μl, 10 μl <strong>and</strong> 5 μl reaction volumes<br />
Reaction <strong>PCR</strong> program<br />
95 °C 95 °C 60 °C melting curve (default<br />
settings)<br />
Lambda 2 min 10 s 40 s only with SYBR<br />
SRY 2 min 10 s 30 s only with SYBR<br />
40 cycles<br />
‡ Table 2: <strong>PCR</strong> programs on the Mastercycler ep realplex 4 S<br />
Application notes<br />
Discussion<br />
The selected assays may indicate that there is a small loss in<br />
sensitivity for the low volume q<strong>PCR</strong> assays. The C values of the<br />
t<br />
10 μl reactions were only slightly higher than those detected for<br />
the 20 μl setups (Figure 1). One reason for the small shift of the Ct value at lower volumes could be explained by the fact that due to<br />
the smaller amount of template used (Table 1), the increase of <strong>PCR</strong><br />
product amount during the course of the reaction was delayed<br />
accordingly. Since the intensity of the detected fluorescence signal<br />
is proportional to the amount of <strong>PCR</strong> product, a delayed increase in<br />
the fluorescence signal at constant instrument detection sensitivity<br />
is observed. For many applications, this small Ct value shift does<br />
not pose a problem. However, this should be taken into account<br />
particularly when working with small amounts of template (low<br />
copy <strong>PCR</strong>).<br />
The reaction efficiencies obtained for all assays shown in Figure 1, in<br />
particular those with low reaction volumes, were comparably good<br />
with values above 0.92.<br />
The reactions presented here were all performed successfully<br />
using the same reagents, concentrations <strong>and</strong> reaction vessels.<br />
The <strong>Eppendorf</strong> twin.tec <strong>PCR</strong> Plate 96 skirted used in this study<br />
has a relatively low total reaction volume of 150 μl/well. Also, by<br />
sealing the plate with an <strong>Eppendorf</strong> Heat Sealing Film, the risk of<br />
evaporation is almost eliminated, which is of particular importance<br />
when working with small reaction volumes. It must be assumed that<br />
not all reaction conditions are equally suited for the establishment<br />
of small reaction volumes. In order to assess this, reduced-volume<br />
<strong>PCR</strong> reactions should be evaluated in direct comparison to larger<br />
volumes, prior to the routine use of small reaction volumes [2].<br />
The integrity of the data fit to the theoretical line of the st<strong>and</strong>ard<br />
curve is described by the r2-coefficient [3].<br />
Lower r2-coefficients of the presented SRY 5 μl setups may indicate<br />
that the lower reaction volumes put higher dem<strong>and</strong>s on liquid<br />
h<strong>and</strong>ling accuracy during <strong>PCR</strong> setup (Figure 1). In order to ensure<br />
reproducible results when routinely working with small volumes, it<br />
is recommended to employ an automated <strong>PCR</strong> setup, such as the<br />
epMotion automated pipetting system. These results demonstrate<br />
that real-<strong>time</strong> <strong>PCR</strong> with small reaction volumes can successfully<br />
be performed on the Mastercycler ep realplex. With the aid of the<br />
epMotion automated pipetting system, up to 75% savings in reagent<br />
costs can be achieved compared with 20 μl real-<strong>time</strong> <strong>PCR</strong> reactions.<br />
References<br />
[1] Leclair B, Sgueglia JB, Wojtowicz PC, Juston AC, Fregeau CJ, Fourney RM. STR DNA typing: increased<br />
sensitivity <strong>and</strong> efficient sample consumption using reduced <strong>PCR</strong> reaction volumes. J Forensic Sci<br />
2003; 48(5):1001-1013.<br />
[2] Thomson E, Vincent B. Reagent volume <strong>and</strong> plate bias in real-<strong>time</strong> polymerase chain reaction.<br />
Analytical Biochemistry 2005; 337: 347-350.<br />
[3] Dorak T (ed.). <strong>Real</strong>-<strong>time</strong> <strong>PCR</strong>. Taylor & Francis Group; 2006.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
73<br />
ARTS | Applications
Applications | ARTS<br />
74<br />
Application notes<br />
<strong>Real</strong>-<strong>time</strong> RT-<strong>PCR</strong> diagnosis of the avian influenza virus<br />
<strong>Real</strong>-<strong>time</strong> RT-<strong>PCR</strong> diagnosis of the avian influenza virus using Mastercycler ® ep<br />
realplex S <strong>and</strong> AIV RT-<strong>PCR</strong> kits from PG Biotech<br />
Ong Wai Kean <strong>and</strong> Christian Rohrer, eppendorf Asia Pacific Headquarters, Malaysia<br />
Abstract<br />
The recent outbreak of avian influenza in different parts of the world<br />
not only has caused major economic losses, but also has presented<br />
a significant threat to public health due to a potential transmission<br />
of the avian influenza virus to humans. The ability to rapidly<br />
recognize avian influenza virus (AIV) in biological specimens is of<br />
utmost importance to enable fast decision making on appropriate<br />
countermeasures to prevent the further spread of the virus. In<br />
this study we evaluated the performance of a commercial Kit, AIV<br />
RT-<strong>PCR</strong> Kit (PG Biotech), for the detection of influenza-specific<br />
RNA using real-<strong>time</strong> reverse transcription <strong>PCR</strong>. The tests were<br />
performed on the Mastercycler ep realplex S (<strong>Eppendorf</strong> <strong>AG</strong>), a<br />
96-well real-<strong>time</strong> <strong>PCR</strong> instrument. It could be demonstrated that the<br />
fast ramping speed of the Mastercycler ep realplex S was able to<br />
significantly shorten the run <strong>time</strong> of the kit as compared to st<strong>and</strong>ard<br />
real-<strong>time</strong> <strong>PCR</strong> instruments. The real-<strong>time</strong> <strong>PCR</strong> Kits could be<br />
implemented on the instrument using a st<strong>and</strong>ard protocol according<br />
to manufacturers specifications with no optimization of parameters<br />
required. With an average Ct value of 24 obtained for the Influenza<br />
A <strong>and</strong> H5 subtype, the combination of the Mastercycler ep<br />
realplex S <strong>and</strong> the AIV RT-<strong>PCR</strong> Kits outperformed the expected<br />
Ct value of 28 stated in the operation manuals of the kits.<br />
Introduction<br />
The highly pathogenic avian influenza virus threatens to become<br />
a worldwide p<strong>and</strong>emic [1] <strong>and</strong> as such, very fast screening <strong>and</strong><br />
detection methods are becoming increasingly important to ensure<br />
that appropriate measures can be taken quickly to contain the<br />
spread of the virus.<br />
Molecular techniques offer a more rapid approach to detecting <strong>and</strong><br />
characterizing the viral genome compared to classical methods.<br />
Many samples can be analyzed in a shorter <strong>time</strong> by performing a<br />
reverse transcription of the virus RNA followed by an AIV specific<br />
<strong>PCR</strong> or real-<strong>time</strong> <strong>PCR</strong> assay.<br />
<strong>PCR</strong> is a specific <strong>and</strong> sensitive technique but samples must be<br />
analyzed via Gel Electrophoresis following the amplification step.<br />
Firstly, this increases the risk of carryover contamination since the<br />
tubes need to be opened <strong>and</strong> secondly, the post <strong>PCR</strong> h<strong>and</strong>ling<br />
steps are <strong>time</strong> consuming <strong>and</strong> cumbersome. Using real-<strong>time</strong> <strong>PCR</strong><br />
offers several advantages over st<strong>and</strong>ard <strong>PCR</strong> when applied for<br />
viral screening purposes. Since result monitoring is integrated in<br />
the process <strong>and</strong> can be done in real <strong>time</strong>, post <strong>PCR</strong> h<strong>and</strong>ling is<br />
not required <strong>and</strong> data can be analyzed while the result is being<br />
generated. This shortens the <strong>time</strong> in obtaining a result considerably.<br />
Also, since real-<strong>time</strong> <strong>PCR</strong> displays broader dynamic range, it is<br />
possible to detect lower copy numbers in a shorter <strong>time</strong> compared<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
to st<strong>and</strong>ard <strong>PCR</strong>. <strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> assays also offer the added<br />
benefit of including several external <strong>and</strong> internal controls which<br />
improves the overall integrity of the assay, thereby reducing the<br />
number of false positive, <strong>and</strong> more importantly, false-negative<br />
results. This coupled with the increased sensitivity that real-<strong>time</strong><br />
<strong>PCR</strong> offers ads to the overall importance that real-<strong>time</strong> <strong>PCR</strong> has<br />
in the modern laboratory.<br />
To satisfy the dem<strong>and</strong> of a diagnostic laboratory, real-<strong>time</strong> <strong>PCR</strong><br />
offers the possibility to screen large numbers of samples very<br />
quickly <strong>and</strong> obtain accurate results at the same <strong>time</strong>. If assays are<br />
run on a fast <strong>and</strong> sensitive real-<strong>time</strong> <strong>PCR</strong> platform the <strong>time</strong> to result<br />
can be shortened even more. <strong>Eppendorf</strong>’s Mastercycler ® ep<br />
realplex S 96 well system with heating rates of 6 deg/sec <strong>and</strong><br />
cooling rates of 4.5 deg/sec allow quick completion of a real-<strong>time</strong><br />
<strong>PCR</strong> assay of 96 samples at one go. The use of 96 individual<br />
LEDs as an excitation source in combination with minimal moving<br />
parts in the optical detection module allow single color fluorescent<br />
detection in 8 seconds which is faster than comparable filter based<br />
real-<strong>time</strong> <strong>PCR</strong> systems.<br />
In this paper, we evaluate the performance of two commercially<br />
available real-<strong>time</strong> RT-<strong>PCR</strong> avian influenza detection kits, AIV A<br />
RT-<strong>PCR</strong> Kit <strong>and</strong> AIV H5 RT-<strong>PCR</strong> Kit (PG Biotech, manufactured for<br />
Qiagen) on the Mastercycler ® ep realplex S.<br />
Overview of avian influenza virus<br />
Influenza viruses have a single-str<strong>and</strong>ed RNA genome <strong>and</strong> are<br />
classified into types A, B or C based on antigenic differences<br />
of their nucleo- <strong>and</strong> matrix proteins. Avian influenza viruses<br />
(AIV) belong to type A, which is able to infect a range of species<br />
including humans, birds, horses <strong>and</strong> pigs. The main antigenic<br />
determinants of influenza A viruses are the haemagglutinin (H) <strong>and</strong><br />
the neuraminidase (N) transmembrane glycoproteins. On the basis<br />
of the antigenicity of these glycoproteins, influenza A viruses are<br />
classified into sixteen H (H1 - H16) <strong>and</strong> nine N (N1 - N9) subtypes.<br />
The subtype H5 of the virus can be transmitted from birds to<br />
human causing major health concerns.<br />
Wild aquatic birds are potential carriers <strong>and</strong> the assumed natural<br />
reservoir of all influenza virus A subtypes [2]. These natural hosts<br />
normally are not seriously affected by an AIV infection but some<br />
domesticated poultry such as chicken or turkey are known to be<br />
particularly susceptible to the infection [3].
<strong>Real</strong>-<strong>time</strong> RT-<strong>PCR</strong> diagnosis of the avian influenza virus<br />
<strong>Real</strong>-<strong>time</strong> RT-<strong>PCR</strong> diagnosis of the avian influenza virus, continued.<br />
Materials <strong>and</strong> Methods<br />
The AIV A RT-<strong>PCR</strong> Kit <strong>and</strong> AIV H5 RT-<strong>PCR</strong> Kit (PG Biotech,<br />
manufactured for Qiagen) are kits that are developed for veterinary<br />
research. The AIV A RT-<strong>PCR</strong> kit detects a gene that is common to<br />
all subtypes of the avian influenza virus <strong>and</strong> the AIV H5 RT-<strong>PCR</strong> kit<br />
detects the hemagglutinin gene of the H5 subtype. Both kits use<br />
hydrolysis probes that have FAM as the reporter dye at the 5‘ end<br />
<strong>and</strong> TAMRA as a quencher at the 3‘ end.<br />
These kits were tested on the Mastercycler ep realplex S<br />
(<strong>Eppendorf</strong>, Germany) which has a 96 well block format, a<br />
gradient function <strong>and</strong> a heating rate of 6 deg/sec <strong>and</strong> cooling<br />
rate of 4.5 deg/sec. Assays were performed with positive <strong>and</strong><br />
negative controls which are included in the kits. The reagents were<br />
dispensed according to the PG Biotech instruction manual. Assays<br />
were run using twin.tec <strong>PCR</strong> plate 96, skirted (<strong>Eppendorf</strong>, Germany)<br />
in combination with Heat Sealing Film (<strong>Eppendorf</strong>, Germany).<br />
The investigation process follows a cascade: the presence of<br />
influenza A specific RNA is detected through a one-step reverse<br />
transcription – real-<strong>time</strong> polymerase chain reaction (RT – real-<strong>time</strong><br />
<strong>PCR</strong>) using primers <strong>and</strong> probes provided in the AIV A RT-<strong>PCR</strong> kit<br />
that are specific for a gene that is common to all subtypes of avian<br />
influenza virus.<br />
When a positive result is obtained, a second one-step RT –<br />
real-<strong>time</strong> <strong>PCR</strong> assay is set up using primers <strong>and</strong> probes from<br />
the AIV H5 RT-<strong>PCR</strong> Kit which are specific for a sequence of the<br />
hemagglutinin gene that is conserved among avian influenza<br />
viruses of subtype H5.<br />
The Mastercycler ep realplex S was programmed according to<br />
manufacturer’s recommendation for block based systems. See<br />
Table 1 for thermal protocols for both kits.<br />
RT-RCR-Thermal Protocol No. of<br />
cycles<br />
‡ Table 1: RT-<strong>PCR</strong> thermal protocol as per manufacturer’s<br />
recommendation<br />
Data acquisition was carried out during the combined annealing/<br />
extension step (*).<br />
Temp<br />
(°C)<br />
Time<br />
Reverse transcription 42 30:00<br />
Initial denaturation 92 3:00<br />
3-step cycling:<br />
Denaturation<br />
Annealing<br />
Extension<br />
2-step cycling:<br />
Denaturation<br />
Annealing/extension*<br />
5<br />
40<br />
92<br />
45<br />
72<br />
92<br />
60<br />
0:10<br />
0:30<br />
1:00<br />
0:10<br />
1:00<br />
Results<br />
Application notes<br />
The positive control with the subtype A primers gave rise to a<br />
signal with an average Ct of 24 cycles (Fig. 1). As for the positive<br />
control with the type H5 primers, the signal has an average Ct of<br />
24.4 cycles. According to PG Biotech, Ct values of approx. 28 are<br />
representative of a good result. The amplification plots for both<br />
AIV A RT-<strong>PCR</strong> Kit <strong>and</strong> AIV H5 RT-<strong>PCR</strong> Kit therefore outperform the<br />
expectations stated in the operation manual of the manufacturer.<br />
Using the Mastercycler ep realplex S Ct values of approx. 24 were<br />
obtained. The tightness of the replicates show good reproducibility<br />
of the results. As expected the negative controls did not show any<br />
amplification.<br />
Performing the AIV A RT-<strong>PCR</strong> Kit <strong>and</strong> AIV H5 RT-<strong>PCR</strong> Kit on the<br />
Mastercycler ep realplex S resulted in a run<strong>time</strong> of only 90 minutes<br />
(Fig. 2). In comparison to st<strong>and</strong>ard real-<strong>time</strong> <strong>PCR</strong> platforms with<br />
slower ramp rates <strong>and</strong> a run<strong>time</strong> of 120 min for the same protocol,<br />
the run-<strong>time</strong> was significantly reduced. Looking at <strong>time</strong> needed<br />
starting from sample preparation to the analysis of the results, real-<br />
<strong>time</strong> <strong>PCR</strong> considerably shortens the <strong>time</strong> needed in comparison to<br />
st<strong>and</strong>ard <strong>PCR</strong>.<br />
Fluorescence (norm)<br />
1 0 0 0<br />
1 0 0<br />
1 0<br />
1<br />
Subtype A<br />
Type H5<br />
0 1 2 3 4 5 6 7 8 9 1 0 1 1 1 2 1 3 1 4 1 5 1 6 1 7 1 8 1 9 2 0 2 1 2 2 2 3 2 4 2 5 2 6 2 7 2 8 2 9 3 0 3 1 3 2 3 3 3 4 3 5 3 6 3 7 3 8 3 9 4 0<br />
Cycle<br />
‡ Figure 1: Amplification plots showing positive identification of<br />
both subtype A <strong>and</strong> H5 genes.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
75<br />
ARTS | Applications
Applications | ARTS<br />
76<br />
Application notes<br />
<strong>Real</strong>-<strong>time</strong> RT-<strong>PCR</strong> diagnosis of the avian influenza virus<br />
<strong>Real</strong>-<strong>time</strong> RT-<strong>PCR</strong> diagnosis of the avian influenza virus, continued.<br />
A<br />
B<br />
C<br />
Sample preparation prior to<br />
RT-<strong>PCR</strong><br />
ca. 2 hrs<br />
RT step<br />
30 min<br />
<strong>PCR</strong><br />
ca 1 hr 30 min<br />
Gel preparation, Electrophoresis<br />
& EtBr Staining<br />
ca. 2 hrs 15 min<br />
<strong>PCR</strong> technique: Total <strong>time</strong> of 6 hours 15 minutes<br />
Sample preparation prior to RT step<br />
q<strong>PCR</strong><br />
RT-<strong>PCR</strong><br />
ca. 2 hrs<br />
30 min ca. 1 hr 30 min<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> with st<strong>and</strong>ard cycler: Total <strong>time</strong> of 4 hours<br />
Sample preparation prior to<br />
RT-<strong>PCR</strong><br />
ca. 2 hrs<br />
RT step<br />
30 min<br />
q<strong>PCR</strong><br />
ca. 1 hr<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> with fast rampling cycler: Total <strong>time</strong> of 3 hours 30 minutes<br />
‡ Figure 2: Expected <strong>time</strong> needed to obtain a result for avian influenza virus detection using <strong>PCR</strong> or real-<strong>time</strong> <strong>PCR</strong><br />
Discussion<br />
The AIV A RT-<strong>PCR</strong> Kit <strong>and</strong> AIV H5 RT-<strong>PCR</strong> Kit show optimal<br />
performance when used on the Mastercycler ep realplex S from<br />
<strong>Eppendorf</strong>. The real-<strong>time</strong> <strong>PCR</strong> Kits to detect avian influenza virus<br />
were successfully implemented on the platform using a st<strong>and</strong>ard<br />
protocol according to manufacturers specifications without any<br />
optimization of parameters.<br />
With average Ct values of 24 obtained for the Influenza A <strong>and</strong> H5<br />
subtype, the combination of the Mastercycler ep realplex <strong>and</strong> the<br />
AIV RT-<strong>PCR</strong> Kits outperforms the expectations of 28 Ct stated<br />
in the operation manuals of the Kits.The fast ramping speed of<br />
the Mastercycler ep realplex real-<strong>time</strong> <strong>PCR</strong> platform was able to<br />
shorten the run <strong>time</strong> as compared to a st<strong>and</strong>ard real-<strong>time</strong> <strong>PCR</strong><br />
instrument, which may need at least two hours to complete the<br />
same assay.<br />
Reduction of <strong>time</strong> was achieved without any need for special<br />
consumables or reagents. At the same <strong>time</strong>, having a 96 well block<br />
format enables a high number of samples to be processed at one<br />
<strong>time</strong>. <strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> is a very fast <strong>and</strong> precise tool that can be<br />
used for rapid avian flu virus detection. Results are obtained in<br />
real <strong>time</strong> without having to do any post <strong>PCR</strong> analysis. Results are<br />
obtained without opening the reaction tubes, which eliminates the<br />
risk of carryover contamination.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
References<br />
[1] Widjaja L, SL Krauss, RJ Webby, X Tao <strong>and</strong> RG Webster. Matrix gene of influenza A viruses isolated<br />
from wild aquatic birds: ecology <strong>and</strong> emergence of influenza A viruses. J.Virol. 78: 8771-8779.<br />
[2] Kaye D <strong>and</strong> CR Pringle. Avian influenza viruses <strong>and</strong> their implication for human health. CID 40:<br />
108-112.<br />
[3] Kamps BS, C Hoffmann <strong>and</strong> W Preiser. Influenza Report 2006.
Improved reproducibility with twin.tec real-<strong>time</strong> <strong>PCR</strong> plates<br />
Improved reproducibility <strong>and</strong> sensitivity in real-<strong>time</strong> <strong>PCR</strong> with <strong>Eppendorf</strong> ® twin.tec<br />
real-<strong>time</strong> <strong>PCR</strong> plates*<br />
Beate Riekens, eppendorf <strong>AG</strong>, Hamburg, Germany<br />
Abstract<br />
In this Application Note, the influence of various consumables<br />
on the results of real-<strong>time</strong> <strong>PCR</strong> is described. In comparison to<br />
transparent micro test tubes, the white wells of the <strong>Eppendorf</strong><br />
twin.tec real-<strong>time</strong> <strong>PCR</strong> plates* result in an improved amplification of<br />
the fluorescence signal <strong>and</strong> a reduced influence of the thermoblock<br />
on the reflection of the signal. These effects in turn lead to<br />
increased reproducibility <strong>and</strong> improved sensitivity in real-<strong>time</strong><br />
<strong>PCR</strong> experiments.<br />
Introduction<br />
In <strong>PCR</strong>, consumables made from polypropylene are mainly used<br />
today, as this material is capable of forming especially thin-walled<br />
<strong>and</strong> well-proportioned micro test tubes that ensure a rapid <strong>and</strong><br />
consistent temperature transfer from the thermoblock to the<br />
sample. In addition, the material is distinguished by low binding<br />
properties with respect to proteins <strong>and</strong> nucleic acids, so that the<br />
reaction components are completely available for efficient <strong>PCR</strong>.<br />
Since the introduction of real-<strong>time</strong> <strong>PCR</strong>, the requirements with<br />
respect to the components have become stricter. This is the case<br />
both for reagents <strong>and</strong> disposables. Transparent micro test tubes,<br />
which are used frequently, can only increase the fluorescence<br />
signal to a limited extent. In addition, due to the permeability<br />
of the material, the fluorescence signal can be reflected from<br />
the thermoblock of the real-<strong>time</strong> <strong>PCR</strong> device, <strong>and</strong> thus have<br />
an interfering influence on the fluorescence signal.<br />
Through the addition of titanium dioxide to the polypropylene,<br />
the fluorescence signal is considerably increased by the reflection<br />
against the walls of white wells. In addition, the fluorescence<br />
signal is enhanced more evenly, because the interfering reflection<br />
of the thermoblock is significantly reduced. This is proven by a<br />
considerable improvement of the reproducibility with respect to<br />
replicate samples, while the background noise of the baseline is<br />
also reduced. This makes it possible to measure an increase in the<br />
fluorescence much earlier, <strong>and</strong> can, depending upon the threshold<br />
value setting, result in lower C t values, amounting to improved<br />
sensitivity of the real-<strong>time</strong> <strong>PCR</strong> experiment.<br />
Materials <strong>and</strong> methods<br />
Application notes<br />
The following plates were evaluated within a comparative<br />
experiment:<br />
‡ <strong>Eppendorf</strong> twin.tec <strong>PCR</strong> plate* with clear wells<br />
‡ <strong>Eppendorf</strong> twin.tec <strong>PCR</strong> plate* with frosted wells<br />
‡ <strong>Eppendorf</strong> twin.tec real-<strong>time</strong> <strong>PCR</strong> plate* with white wells<br />
‡ Competitor plate A with white wells<br />
‡ Competitor plate B with white wells<br />
For the comparison of the various plates, all preparations were<br />
pipetted with the same automated pipetting station epMotion ®<br />
5070, <strong>and</strong> were processed with the same real-<strong>time</strong> <strong>PCR</strong> system<br />
Mastercycler ® ep realplex 4 S. The following <strong>PCR</strong> system was used<br />
in a SYBR Green application:<br />
<strong>PCR</strong> target: 108 bp, fragment from lambda DNA<br />
Forward primer (600 nM): cgcacaggaactgaagaatg,<br />
Reverse primer (300 nM): ccgtcgagaatactggcaat,<br />
Template: lambda DNA (Roche)<br />
A tenfold dilution series of the lambda DNA was manually created<br />
for a range of 100 – 1x10 7 copies for each reaction preparation. In<br />
order to exclude the influence of potential pipetting inaccuracies,<br />
all additional components were added to the various DNA<br />
concentrations.<br />
These mini-master mixes were pipetted into the respective wells of<br />
a plate in 6 replicates of 20 μl each with the help of the <strong>Eppendorf</strong><br />
epMotion 5070. The plates were then heat-sealed with <strong>Eppendorf</strong><br />
Heat Sealing Film in order to prevent evaporation. Following this,<br />
the plates were centrifuged for 1 min at 500 x g <strong>and</strong> real-<strong>time</strong> <strong>PCR</strong><br />
was carried out with the following program:<br />
95°C 2 min<br />
95°C 10s<br />
60°C 30s<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
40x<br />
*<strong>Eppendorf</strong> owns protective rights under European Patent EP 1 161 994, US Patent 7,347,977.<br />
email: info@eppendorf.com • www.eppendorf.com<br />
77<br />
ARTS | Applications
Applications | ARTS<br />
78<br />
Application notes<br />
Improved reproducibility with twin.tec real-<strong>time</strong> <strong>PCR</strong> plates<br />
Improved reproducibility with twin.tec real-<strong>time</strong> <strong>PCR</strong> plates, continued.<br />
a)<br />
‡ Figure 1: Comparison of fluorescence signals <strong>and</strong> reproducibility of replicates<br />
a) A lambda serial dilution of 100 to 1x10 7 copies per reaction was amplified with SYBR Green in twin.tec <strong>PCR</strong> plate* with frosted wells (blue)<br />
<strong>and</strong> twin.tec real-<strong>time</strong> <strong>PCR</strong> plate* with white wells (red). b) The exponential phase of 6 replicates each (1000 – 1x10 6 copies) are displayed in<br />
enlarged amplification plots.<br />
Results <strong>and</strong> discussion<br />
In comparison to a plate with frosted wells (semi-transparent),<br />
the absolute fluorescence signals in the twin.tec real-<strong>time</strong> <strong>PCR</strong><br />
plates* are strengthened more than tenfold due to the reflective<br />
properties of titanium dioxide (Fig. 1a). Equally good signal<br />
improvements could also be observed in comparison to clear<br />
wells (data not shown). In addition to the considerably stronger<br />
signals, the enlarged amplification plots (Fig. 1b) show that the<br />
replicates of the respective DNA concentrations are amplified<br />
much more homogeneously than in transparent wells. In these,<br />
the fluorescence is reflected not only onto the walls of the wells,<br />
but also onto the thermoblock. In addition, in the event that the<br />
reaction vessel is not evenly <strong>and</strong> completely in contact with the<br />
thermoblock, the fluorescence signal will be additionally dispersed<br />
due to the refraction index of air.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
b)<br />
This influence is prevented by the white wells of the twin.tec<br />
real-<strong>time</strong> <strong>PCR</strong> plates*.<br />
For purposes of most effective comparability with various<br />
consumables, the st<strong>and</strong>ard deviations for each 6 replicates were<br />
averaged over several log steps <strong>and</strong> compared (Fig. 2).<br />
st<strong>and</strong>ard deviation (mean over 4 logs)<br />
0,1<br />
0,08<br />
0,06<br />
0,04<br />
0,02<br />
0<br />
‡ Figure 2: Mean st<strong>and</strong>ard deviation over a range of 4 logs<br />
The st<strong>and</strong>ard deviation of 6 replicates each was calculated at 1000<br />
to 1x10 6 copies per reaction at a <strong>time</strong>. The values were averaged<br />
afterwards.<br />
Plate 1 Plate 2 Plate 3<br />
twin.tec <strong>PCR</strong> Plate, clear wells*<br />
twin.tec <strong>PCR</strong> Plate, frosted wells*<br />
twin.tec real-<strong>time</strong> <strong>PCR</strong> Plate, white wells*<br />
Supplier A, white wells<br />
Supplier B, white wells<br />
Compared plates<br />
Plate 4 Plate 5
Improved reproducibility with twin.tec real-<strong>time</strong> <strong>PCR</strong> plates<br />
Improved reproducibility with twin.tec real-<strong>time</strong> <strong>PCR</strong> plates, continued.<br />
ct shift compared to clear wells<br />
–1,0<br />
–0,8<br />
–0,6<br />
–0,4<br />
–0,2<br />
0<br />
twin.tec <strong>PCR</strong> plate, frosted wells*<br />
twin.tec real-<strong>time</strong> <strong>PCR</strong> plate, white wells*<br />
1,00E+07<br />
1,00E+06 1,00E+05 1,00E+04<br />
‡ Figure 3: C t shift improvement<br />
C t values which were obtained in twin.tec <strong>PCR</strong> plates* with clear<br />
wells were set as equal to 1 for all examined DNA concentrations.<br />
The Ct shift improvement of all other plates was compared to the<br />
twin.tec <strong>PCR</strong> plates* with clear wells.<br />
copies / reaction<br />
Supplier A, white wells<br />
Supplier B, white wells<br />
1,00E+03 1,00E+02<br />
While the transparent wells have a mean st<strong>and</strong>ard deviation of 0.09<br />
<strong>and</strong> 0.1, the reproducibility of the replicates can be improved with<br />
the twin.tec real-<strong>time</strong> <strong>PCR</strong> plates* to a st<strong>and</strong>ard deviation of less<br />
than 0.04. White plates of other manufacturers also reduced the<br />
st<strong>and</strong>ard deviation, down to 0.05. The consistent enhancement of<br />
the fluorescence signal by the white polypropylene also improves<br />
the signal-to-noise ratio of the measurement, thus supporting<br />
the earlier differentiation of baseline <strong>and</strong> point of increasing<br />
fluorescence. The determination of Ct values generally takes place<br />
in the exponential increase of the amplification curve. The threshold<br />
value for the determination of Ct values is thus very often set to<br />
Application notes<br />
the default of ten-fold st<strong>and</strong>ard deviation of the baseline. This<br />
evaluation therefore requires a qualitatively good baseline with a low<br />
noise level. The comparison shown in figure 3 was carried out with<br />
the help of this threshold value setting. It was thereby shown that the<br />
plate with clear wells generated the highest C t values. These were<br />
set as equal to 1 for all examined DNA concentrations <strong>and</strong> viewed<br />
in relation to the C t shift of all other tested plates.<br />
It thereby became clear that frosted wells also offer a minor<br />
improvement of the C t values in comparison to completely clear<br />
wells. In contrast, white wells improve the C t values for all DNA<br />
concentrations by up to 0.92. This increases the sensitivity of the<br />
assay by a factor of nearly 2, assuming an amplification efficiency<br />
of 100%. The white plates of other manufacturers also show an<br />
improvement of the Ct values in comparison to clear wells. However,<br />
the Ct shift of alternative white well plates lies at a maximum of 0.77<br />
<strong>and</strong> 0.54, respectively.<br />
Conclusion<br />
The use of <strong>Eppendorf</strong> twin.tec real-<strong>time</strong> <strong>PCR</strong> plates* can increase<br />
the sensitivity <strong>and</strong> the reproducibility of real-<strong>time</strong> <strong>PCR</strong> experiments.<br />
This offers the greatest advantage for real-<strong>time</strong> <strong>PCR</strong> systems with<br />
low fluorescence or with small reaction volumes, which can lead to<br />
a reduction of the signal. As a result of the improvements shown<br />
here, the use of white wells can be of advantage in the analysis of<br />
samples, especially those with low nucleic acid concentrations.<br />
*<strong>Eppendorf</strong> owns protective rights under European Patent EP 1 161 994, US Patent 7,347,977.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
79<br />
ARTS | Applications
Applications | ARTS<br />
80<br />
Application notes<br />
Optimizing q<strong>PCR</strong> with small reaction volumes<br />
Successful q<strong>PCR</strong> with small reaction volumes on <strong>Eppendorf</strong> Mastercycler ® ep realplex<br />
Cynthia Potter, <strong>Eppendorf</strong> UK Limited; Arun Kumar, Ph.D., <strong>Eppendorf</strong> North America<br />
Abstract<br />
Recent technological developments in the field of q<strong>PCR</strong> enable<br />
short run <strong>time</strong>s <strong>and</strong> improved reproducibility. The objective of this<br />
study was to assess the reproducibility of q<strong>PCR</strong> data with 5 µl<br />
reaction volumes on <strong>Eppendorf</strong> Mastercycler ep realplex.<br />
Introduction<br />
Methodologies in genomics are expensive, <strong>and</strong> there is a critical<br />
need to lower costs by minimizing reagent use. One of the major<br />
cost components of the q<strong>PCR</strong> technique is reagent cost, including<br />
a mastermix that contains dNTPs, <strong>PCR</strong> enzymes (Taq), Mg 2+ <strong>and</strong><br />
stabilizers. A second costly component is the template: the DNA<br />
or RNA is often in short supply <strong>and</strong>/or expensive as well as <strong>time</strong>consuming<br />
to extract <strong>and</strong> purify; a 25 μl reaction volume would be<br />
prohibitive in this case. Another challenge of q<strong>PCR</strong> is to minimize<br />
the assay variability. Bustin [1] has demonstrated significant<br />
user pipetting variability, providing reasons for the use of automated<br />
liquid h<strong>and</strong>ling stations in achieving greater assay reproducibility.<br />
The solution to keeping reagent costs down is to lower reaction<br />
volume while maintaining the same ratios of reaction components.<br />
However, many problems can arise in low-volume reactions,<br />
which can then lead to <strong>PCR</strong> efficiency plummeting to unacceptable<br />
levels for quantification. Perhaps the most significant problem with<br />
small reaction volumes is evaporation off the walls of the wells—<br />
the reagents are pulled out of the reaction, leaving variable<br />
concentrations of all reactants. To control volume variation<br />
due to pipetting error, ROX normalization is some<strong>time</strong>s used.<br />
However, concentration of key reactants—rather than volume—<br />
remains a challenge in terms of reproducibility, because variability<br />
of evaporation in low-volume reactions cannot be controlled <strong>and</strong><br />
evaporation levels are not reproducible. Controls such as ROX<br />
cannot normalize for this. Additionally, on instruments that perform<br />
q<strong>PCR</strong> in 1.5 to 2 hours, this evaporation cannot be completely<br />
eliminated. The amount of heat being applied is too great for<br />
excessive periods of <strong>time</strong>; therefore, it is imperative that run<br />
<strong>time</strong>s are short when using low-volume reactions.<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
<strong>Eppendorf</strong> offers the Mastercycler ep realplex line of quantitative<br />
real-<strong>time</strong> <strong>PCR</strong> instruments to the research community. In this<br />
article, data is presented that demonstrate robust quantitative <strong>PCR</strong><br />
of three mouse genes <strong>and</strong> Lambda DNA, using SYBR ® Green I <strong>and</strong><br />
TaqMan ® , in a total reaction volume of 5 μl.<br />
<strong>Eppendorf</strong> epMotion ® 5070, an automated pipetting system, has<br />
been used in academic <strong>and</strong> pharmaceutical research communities<br />
for small-volume assay setup to increase throughput <strong>and</strong> achieve<br />
significant cost reduction. The use of a liquid h<strong>and</strong>ling workstation<br />
also addresses the human error in reaction assembly, ensuring<br />
assay accuracy <strong>and</strong> reproducibility.<br />
This article will show that by combining assay setup with an<br />
automated pipetting system <strong>and</strong> a sensitive <strong>and</strong> fast real-<strong>time</strong> <strong>PCR</strong><br />
instrument, q<strong>PCR</strong> reactions in the 5 μl range are easily achieved. In<br />
addition, the <strong>PCR</strong> efficiencies <strong>and</strong> correlation coefficients are nearly<br />
identical to reactions performed at higher volumes.
Optimizing q<strong>PCR</strong> with small reaction volumes<br />
Successful q<strong>PCR</strong> with small reaction volumes on <strong>Eppendorf</strong> Mastercycler ® ep realplex, continued.<br />
Methods<br />
Quantitative real-<strong>time</strong> <strong>PCR</strong> experiments<br />
All experiments were run on an <strong>Eppendorf</strong> Mastercycler ep<br />
realplex 4 S with a silver block. A mastermix containing 5 PRIME<br />
<strong>Real</strong>MasterMix*, SYBR ® Green I assays <strong>and</strong> primers was prepared<br />
(to amplify <strong>and</strong> detect Beta actin) as well as two proprietary target<br />
genes for mouse <strong>and</strong> a 104 bp sequence of Lambda DNA. A<br />
mastermix containing 5 PRIME <strong>Real</strong>MasterMix Probe ROX , primers<br />
<strong>and</strong> CAL Fluor ® Gold 540/Black Hole Quencher-1 probe<br />
(Biosearch Technologies) was prepared for Lambda. All reactions<br />
were set up on the <strong>Eppendorf</strong> epMotion ® 5070 <strong>and</strong> dispensed into<br />
<strong>Eppendorf</strong> ® twin.tec 96-well skirted <strong>PCR</strong> plates. The plates were<br />
then sealed with <strong>Eppendorf</strong> Heat Sealing Film, using an <strong>Eppendorf</strong><br />
Heat Sealer. For comparison, parallel reactions were assembled<br />
with 20 μl reaction volumes.<br />
Assay setup — SYBR Green I assays<br />
Each q<strong>PCR</strong> reaction was run in triplicate. A three-fold dilution series<br />
was prepared down to ~142.5 copies of the human genes studied.<br />
A two-step protocol (denature/cycling) was followed that consisted of<br />
initial denaturation of 2 min, followed by 2 s secondary denaturing<br />
at 95 ºC <strong>and</strong> annealing/extension at ~60 ºC (depending on gradient<br />
optimization for each amplicon), cycled 40 <strong>time</strong>s.<br />
*U.S. Pat. 6,667,165<br />
1000<br />
Fluorescence (norm)<br />
100<br />
10<br />
Application notes<br />
1<br />
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40<br />
Cycle<br />
Threshold: 393 (Adjusted manually)<br />
Baseline settings: automatic, Drift correction OFF<br />
40<br />
Ct[Cycle]<br />
35<br />
30<br />
25<br />
20<br />
15<br />
Slope: -3.552<br />
Y-Intercept: 37.89<br />
Efficiency: 0.91<br />
R^2: 0.998<br />
10 1000 1.0E+05 1.0E+07<br />
Amount[Copies]<br />
‡ Fig. 1: TaqMan ® assay with CAL FLUOR Gold 540<br />
Top: Reproducibility with 5 μl total reaction volume per well.<br />
Amplification plot (log view) of 104 bp amplicon for Lambda DNA<br />
target. A 10-fold dilution series of Lambda DNA (Promega ® ) was<br />
prepared as above <strong>and</strong> amplified with 5 PRIME <strong>Real</strong>MasterMix<br />
Probe. Forward <strong>and</strong> reverse primers were used at 400 nM each,<br />
while CFG540/BHQ-1 was used at 400 nM, 50 cycles, 43 min.<br />
Threshold: 393 (Adjusted manually)<br />
Baseline settings: automatic, Drift correction OFF<br />
Bottom: St<strong>and</strong>ard curve generated with data from<br />
above TaqMan assay<br />
Results: Slope=–3.552, Y-Intercept=37.89, Efficiency=0.91,<br />
R2 =0.998<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
81<br />
ARTS | Applications
Applications | ARTS<br />
82<br />
Application notes<br />
10000<br />
Fluorescence (norm)<br />
1000<br />
100<br />
10<br />
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40<br />
Cycle<br />
Threshold: 2334 (Noiseb<strong>and</strong>)<br />
Baseline settings: automatic, Drift correction OFF<br />
35<br />
Ct[Cycle]<br />
30<br />
25<br />
20<br />
15<br />
10<br />
5.0<br />
Slope: -3.591<br />
Y-Intercept: 35.61<br />
Efficiency: 0.90<br />
R^2: 1.000<br />
Optimizing q<strong>PCR</strong> with small reaction volumes<br />
Successful q<strong>PCR</strong> with small reaction volumes on <strong>Eppendorf</strong> Mastercycler ® ep realplex, continued.<br />
10 1000 1.0E+05<br />
Amount[Copies]<br />
1.0E+07<br />
‡ Fig. 2: SYBR ® Green I assay<br />
Top: Reproducibility with 5 μl total reaction volume per well.<br />
Amplification plot (log view) of 104 bp amplicon for Lambda DNA<br />
target. A 10-fold dilution series of Lambda DNA (Promega ® ) was<br />
prepared as above <strong>and</strong> amplified with 5 PRIME <strong>Real</strong>MasterMix with<br />
SYBR Green I. Forward <strong>and</strong> reverse primers were used at 400 nM<br />
each, 50 cycles, 43 min.<br />
Threshold: 2,334 (Noiseb<strong>and</strong>)<br />
Baseline settings: automatic, Drift correction OFF<br />
Bottom: St<strong>and</strong>ard curve generated with data from<br />
above SYBR Green assay<br />
Results: Slope=–3.591, Y-Intercept=35.61, Efficiency=0.90,<br />
R 2 =1.000<br />
TaqMan ® assay with CAL Fluor ® Gold 540<br />
The following amplifications were performed from a 10-fold<br />
dilution series prepared from Lambda DNA (Promega) at a range<br />
from 1.42 x 10 7 copies down to 142.5 copies. A two-step protocol<br />
(denature/extension) was followed that consisted of initial denaturation<br />
of 2 min, followed by 2 s secondary denaturing at 95 °C <strong>and</strong><br />
annealing/extension at 60 °C.<br />
1000<br />
Fluorescence (norm)<br />
100<br />
10<br />
1<br />
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40<br />
Cycle<br />
Threshold: 96 (Noiseb<strong>and</strong>)<br />
Baseline settings: automatic, Drift correction ON<br />
Ct[Cycle]<br />
34<br />
32<br />
30<br />
28<br />
26<br />
24<br />
22<br />
20<br />
Slope: -3.309<br />
Y-Intercept: 34.96<br />
Efficiency: 1.01<br />
R^2: 0.994<br />
10 1000<br />
Amount[Copies]<br />
‡ Fig. 3: SYBR Green I assay<br />
Top: Reproducibility with 5 μl total reaction volume per well.<br />
Amplification plot of 69 bp G4 (proprietary) target in triplicate. A<br />
three-fold dilution series of mouse genomic DNA (Promega) was<br />
prepared <strong>and</strong> amplified with 5 PRIME <strong>Real</strong>MasterMix with SYBR<br />
Green I. Forward <strong>and</strong> reverse primers were used at 300 nM each,<br />
45 cycles, 41 min.<br />
Threshold: 96 (Noiseb<strong>and</strong>)<br />
Baseline settings: automatic, Drift correction ON<br />
Bottom: St<strong>and</strong>ard curve generated with data from<br />
above SYBR Green assay<br />
Results: Slope=–3.309, Y-Intercept=34.96, Efficiency=1.01,<br />
R 2 =0.994<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Results<br />
The TaqMan assay was linear over 6 orders of magnitude in a 5 μl<br />
reaction setup. In the SYBR Green I assay, there was amplification in<br />
the “no template” controls representing primer-dimer (melting curve<br />
not shown). SYBR Green I cannot reliably quantify at the singlecopy<br />
level due to the inherent background of this assay; however,<br />
7 logs of quantification were achieved—down to ~14 copies.
10000<br />
Fluorescence (norm)<br />
1000<br />
100<br />
10<br />
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40<br />
Cycle<br />
Threshold: 1568 (Adjusted manually)<br />
Baseline settings: automatic, Drift correction ON<br />
34<br />
Ct[Cycle]<br />
32<br />
30<br />
28<br />
26<br />
24<br />
22<br />
20<br />
Slope: -3.207<br />
Y-Intercept: 34.13<br />
Efficiency: 1.05<br />
R^2: 0.996<br />
Optimizing q<strong>PCR</strong> with small reaction volumes<br />
Successful q<strong>PCR</strong> with small reaction volumes on <strong>Eppendorf</strong> Mastercycler ® ep realplex, continued.<br />
10 1000<br />
Amount[Copies]<br />
‡ Fig. 4: SYBR ® Green I assay<br />
Top: Reproducibility with 5 μl total reaction volume per well.<br />
Amplification plot of 71 bp G1 (proprietary) target in triplicate. A<br />
three-fold dilution series of mouse genomic DNA (Promega ® ) was<br />
prepared <strong>and</strong> amplified with 5 PRIME <strong>Real</strong>MasterMix with SYBR<br />
Green I. Forward <strong>and</strong> reverse primers were used at 300 nM each,<br />
45 cycles, 41 min.<br />
Threshold: 1,568 (adjusted manually)<br />
Baseline settings: automatic, Drift correction ON<br />
Bottom: St<strong>and</strong>ard curve generated with data from<br />
above SYBR Green assay<br />
Results: Slope=–3.207, Y-Intercept=34.13, Efficiency=1.05,<br />
R2 =0.996<br />
Application notes<br />
Conclusions<br />
Highly reproducible results were obtained with a small (5 μl)<br />
reaction volume (Figs. 1–4); <strong>and</strong> nearly identical <strong>PCR</strong> efficiencies<br />
<strong>and</strong> correlation coefficients were obtained with 5 μl reaction<br />
volumes as compared with 20 μl reaction volumes (not shown).<br />
Working with low volumes presents challenges due to evaporation,<br />
but these challenges are overcome by using a fast (silver) block,<br />
which can easily run 40 cycles in 25 minutes. These initial runs<br />
were ~40 min for 45–50 cycles.<br />
Mastercycler ep realplex achieves uniform heating across the block<br />
through its Triple Circuit Technology, which features six Peltier<br />
elements to ensure this precise temperature control. In addition,<br />
the 96-LED array excitation source is positioned above the block<br />
so that each well is maximally <strong>and</strong> uniformly excited. Other cyclers<br />
that utilize bulbs as a light source above the plate <strong>and</strong> in the middle<br />
exhibit edge effects with 25 μl reactions; these edge effects may be<br />
exacerbated when dropping to small volumes, which, consequently,<br />
may not achieve the same level of uniformity required for success<br />
when using low-volume reactions. To ensure that all photons are<br />
captured, realplex’s 96 fiber-optic cables effectively capture the<br />
emission from each well <strong>and</strong> pass it into channel photo-multiplier<br />
tubes. To date, these are the most sensitive detectors available.<br />
We believe the combination of optical sensitivity, excitation <strong>and</strong><br />
emission—plus the uniformity of heating—are responsible for the<br />
reproducible q<strong>PCR</strong> reactions at low volumes on the Mastercycler<br />
ep realplex. In addition, short run <strong>time</strong>s also resolve the issue of<br />
sample evaporation from the wells.<br />
The ease of use for reaction setup with the epMotion ® 5070<br />
workstation <strong>and</strong> the advanced features of Mastercycler ep<br />
realplex enable reaction volumes to be scaled down to 5 μl. This<br />
is an 80% reduction in volume—when compared with the typical<br />
25 μl reaction volume—<strong>and</strong> represents substantial reagent<br />
cost-savings.<br />
There is a clear advantage to saving reagent cost when working<br />
in high-throughput gene expression analysis. Saving <strong>time</strong> is also<br />
advantageous, <strong>and</strong> the speed of the realplex silver block allows the<br />
completion of a 40-cycle q<strong>PCR</strong> reaction in as little as 23 minutes.<br />
The combination of the epMotion 5070 <strong>and</strong> Mastercycler ep<br />
realplex provides these <strong>time</strong>- <strong>and</strong> cost-savings.<br />
Reference<br />
[1] Bustin SA. Quantification of mRNA using real-<strong>time</strong> reverse transcription <strong>PCR</strong> (RT-<strong>PCR</strong>): trends <strong>and</strong><br />
problems. J. Endocrinology. 2002;29:23-39.<br />
*U.S. Pat. 6,667,165.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
83<br />
ARTS | Applications
Applications | ARTS<br />
84<br />
Application notes<br />
Additional application notes on the Web<br />
Visit www.eppendorfna.com/arts regularly to view <strong>and</strong> download additional<br />
<strong>Automation</strong> <strong>and</strong> <strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> application notes. Below is a sampling of what<br />
you can find on our automated pipetting systems. The site is continually updated<br />
as new applications are added, so be sure to check back frequently!<br />
epMotion ® 5070<br />
High-throughput, fully automated real-<strong>time</strong> <strong>PCR</strong> diagnostics of HBV<br />
<strong>and</strong> salmonella<br />
<strong>PCR</strong> product purification using the <strong>Eppendorf</strong> epMotion 5070 liquid<br />
h<strong>and</strong>ling workstation together with the 5 PRIME Perfectprep ® <strong>PCR</strong><br />
Cleanup 96 kit<br />
Minimization of remaining volumes in plates <strong>and</strong> tubes<br />
Facilitating <strong>PCR</strong> setup via an automated liquid h<strong>and</strong>ling system<br />
Loading the Invitrogen ® E-Gels with the epMotion 5070<br />
epMotion 5075<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Automated isolation of plant genomic DNA using ChargeSwitch<br />
technology<br />
Reproducible <strong>and</strong> easy automated purification of plant<br />
genomic DNA<br />
Isolation of high-quality plasmid DNA using the Perfectprep Plasmid<br />
96 VAC Direct Bind kit on the epMotion 5075 VAC workstation<br />
Isolation of high-quality BAC DNA using the Perfectprep BAC 96 kit<br />
on the epMotion 5075 VAC workstation<br />
Purification of <strong>PCR</strong> products using the Perfectprep <strong>PCR</strong> Cleanup<br />
96 kit on the epMotion 5075 VAC workstation<br />
Guideline for processing the RNeasy ® 96 BioRobot ® 8000 kit on the<br />
epMotion 5075 VAC workstation<br />
Guidelines for processing the QIAamp ® DNA Blood BioRobot MDx<br />
kit on the epMotion 5075 VAC workstation<br />
High-throughput RNA preparation using the QI<strong>AG</strong>EN ® RNeasy 96<br />
BioRobot 8000 kit on the workstation epMotion 5075
Picture: Model of a Taq DNA polymerase with a DNA str<strong>and</strong>. Image made with Molsoft ® -ICM. www.molsoft.com<br />
Appendix<br />
ARTS<br />
85
TOC | Appendix<br />
86<br />
Info<br />
Appendix Table of Contents<br />
<strong>Eppendorf</strong> ARTS is dedicated to helping you perform successful<br />
real-<strong>time</strong> <strong>PCR</strong> experiments. This section covers q<strong>PCR</strong> basics <strong>and</strong><br />
the various detection chemistries used, <strong>and</strong> we include a list of<br />
relevant websites where you can get more information about<br />
q<strong>PCR</strong> <strong>and</strong>/or designing optimized q<strong>PCR</strong> primers.<br />
Description Page<br />
Definitions, concepts <strong>and</strong> overview of real-<strong>time</strong> quantitative <strong>PCR</strong> principles 87<br />
Background information 87<br />
Advantages of q<strong>PCR</strong> over traditional endpoint <strong>PCR</strong> 88<br />
Detection chemistries 88<br />
Double-str<strong>and</strong>ed DNA-binding dyes 88<br />
Probe-base chemistries 89<br />
Methods of real-<strong>time</strong> <strong>PCR</strong> quantification 90<br />
Absolute quantification (st<strong>and</strong>ard curve method) 90<br />
Relative quantification (comparative C t method) 90<br />
Methods of primer <strong>and</strong> probe validation 91<br />
Optimization of forward <strong>and</strong> reverse primer concentrations 91<br />
Primer <strong>and</strong> probe validation: general strategy for new q<strong>PCR</strong> assay development 91<br />
References, resources <strong>and</strong> useful websites 92<br />
Calculating primer quantity 93<br />
<strong>PCR</strong> calculator 94<br />
Abbreviations, symbols <strong>and</strong> conversion factors 96<br />
Genetic code <strong>and</strong> amino acid properties 98<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.
Definitions, concepts <strong>and</strong> overview of real-<strong>time</strong> quantitative <strong>PCR</strong> principles<br />
What is real-<strong>time</strong> quantitative <strong>PCR</strong>?<br />
<strong>Real</strong>-<strong>time</strong> quantitative <strong>PCR</strong>, or “q<strong>PCR</strong>,” is a technique that reaches<br />
far beyond the limitations of st<strong>and</strong>ard <strong>PCR</strong>. It provides a unique,<br />
multidimensional perspective of a gene’s presence, its function—<br />
even its regulation—in a concrete, quantifiable manner.<br />
What are its applications/uses?<br />
The applications of real-<strong>time</strong> <strong>PCR</strong> technology are a testament to<br />
its wide range of influence, <strong>and</strong> they include the analysis of gene<br />
expression <strong>and</strong> gene regulation, determinations of the effects of<br />
variations in genetic composition, <strong>and</strong> the identification <strong>and</strong><br />
quantification of microorganisms <strong>and</strong> viruses, among others.<br />
<strong>PCR</strong> can be used to estimate the amount of a particular target<br />
DNA or RNA in a sample relative to either a st<strong>and</strong>ard or another<br />
sample that has been subjected to some treatment or <strong>time</strong><br />
progression—but it has very limited value as a quantitative<br />
tool. <strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> exp<strong>and</strong>s the utility of <strong>PCR</strong> for quantitation<br />
through the incorporation of a fluorescent reporter molecule—or<br />
molecules—to each assay. Fluorescent intensity, which increases<br />
proportionally with each DNA molecule amplified, is measured<br />
during each cycle of <strong>PCR</strong> <strong>and</strong> plotted over <strong>time</strong>. As there is a direct<br />
correlation between the amount of <strong>PCR</strong> product in each cycle <strong>and</strong><br />
the progression of a fluorescence amplification curve, an analysis<br />
of this curve enables calculation of the original starting quantity of<br />
target DNA or RNA.<br />
Background information<br />
Quantitative <strong>PCR</strong> is the most rapid <strong>and</strong> sensitive quantitative<br />
method for target RNA or DNA, combining the extraordinary<br />
sensitivity of the <strong>PCR</strong> process with highly sensitive optical<br />
detection technology. The fluorescence generated by a sample<br />
of DNA or RNA during real-<strong>time</strong> <strong>PCR</strong> is plotted over <strong>time</strong> or,<br />
rather, <strong>PCR</strong> cycle number. The middle of its curve represents<br />
the exponential phase of <strong>PCR</strong>—when the levels of generated<br />
fluorescence exceed background fluorescence, but reagents<br />
have not nearly begun to reach limiting factors.<br />
Each sample or reaction is assigned a specific value in real-<strong>time</strong><br />
<strong>PCR</strong>, referred to as cycle threshold (C t)—the point or cycle number<br />
at which the fluorescence curve for that sample exceeds back-<br />
ground fluorescence <strong>and</strong> measurements become meaningful.<br />
Samples with the highest starting target amount will also have the<br />
highest values of amplified target in a given <strong>PCR</strong> cycle number.<br />
This means their fluorescence curves will exceed background<br />
earlier <strong>and</strong> cross the threshold at an earlier cycle number; thus, the<br />
more abundant the starting quantity of template or target, the lower<br />
the C t value of that sample.<br />
There are certain assumptions that are made about the sample<br />
C t value <strong>and</strong> initial target amount: early on in a <strong>PCR</strong> reaction <strong>and</strong><br />
into the exponential phase, there is a doubling of fluorescence<br />
from one cycle to the next that is directly proportional to the<br />
doubling of amplicons; therefore, when a sample provides<br />
known values—a C t value (cycle number to reach threshold) <strong>and</strong><br />
a fluorescence value—starting sample quantity can be calculated<br />
from the knowledge that at each earlier cycle number, exactly half<br />
the quantity of target was present.<br />
Due to the exponential nature of <strong>PCR</strong> amplification, we can<br />
extrapolate that in the event there are 80 copies of a gene present<br />
during cycle number 4: cycle number 3 had 40 copies, cycle<br />
number 2 had 20 copies <strong>and</strong> cycle number 1 had 10; <strong>and</strong> at<br />
<strong>time</strong>-point zero, we began with just 5 copies in the sample—<br />
an exceptionally sensitive measurement of a gene’s quantity.<br />
The plateau stage of any real-<strong>time</strong> <strong>PCR</strong> curve represents the<br />
endpoint of that reaction—the point at which there is significant<br />
depletion of one or more reaction components. At the plateau<br />
stage the amplification curves of real-<strong>time</strong> <strong>PCR</strong> are no longer<br />
exponential, which means that the association of a two-fold<br />
increase in quantity from one cycle number to the next has come<br />
to an end. For this reason, real-<strong>time</strong> <strong>PCR</strong> is not concerned with<br />
plateau endpoints (Fig. 1).<br />
‡ Fig. 1: <strong>PCR</strong>—kinetic vs. endpoint detection<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
Info<br />
A plot of the quantity of amplicon DNA over <strong>time</strong>; in real-<strong>time</strong> <strong>PCR</strong><br />
we are only concerned with amplification during the exponential<br />
phase of amplification, as accurate quantification of DNA is not<br />
possible at the plateau.<br />
email: info@eppendorf.com • www.eppendorf.com<br />
87<br />
Appendix | <strong>Real</strong>-<strong>time</strong> q<strong>PCR</strong>
<strong>Real</strong>-<strong>time</strong> q<strong>PCR</strong> | Appendix<br />
88<br />
Info<br />
Definitions, concepts <strong>and</strong> overview of real-<strong>time</strong> quantitative <strong>PCR</strong> principles<br />
Advantages of q<strong>PCR</strong> over traditional endpoint <strong>PCR</strong><br />
In addition to the capacity for highly sensitive detection, real-<br />
<strong>time</strong> <strong>PCR</strong> can provide quantitative data across a wide dynamic<br />
range—on Mastercycler ® ep realplex, detection from single<br />
molecules to up to 10 9 molecules of the same target sequence is<br />
possible in a single experiment. Furthermore, there is no additional<br />
h<strong>and</strong>ling required for these samples—<strong>and</strong> no <strong>time</strong> or money is<br />
wasted on gel analysis with an end result that is semiquantitative,<br />
at best. Adding to its convenience <strong>and</strong> speed, amplification <strong>and</strong><br />
detection occur simultaneously, making q<strong>PCR</strong> technology a highly<br />
efficient alternative.<br />
Detection chemistries<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> detection can be performed with one of many<br />
available fluorescent reporters, including sequence-specific,<br />
dual-labeled probes such as TaqMan ® , molecular beacons,<br />
hybridization or locked nucleic acid (LNA) probes. Alternatively,<br />
free dyes that bind to double-str<strong>and</strong>ed DNA (dsDNA), such as<br />
SYBR ® Green I, can also be used.<br />
Double-str<strong>and</strong>ed DNA-binding dyes<br />
DNA-binding dyes like SYBR Green I are a relatively convenient<br />
<strong>and</strong> inexpensive option for real-<strong>time</strong> <strong>PCR</strong> chemistries, as they<br />
are simply added to a <strong>PCR</strong> reaction mix—no sequence-specific,<br />
synthetic oligonucleotides other than primers need to be prepared.<br />
Free in solution, DNA-binding dyes exhibit low levels of background<br />
fluorescence until they find their primary match; when they bind<br />
to the minor groove of their double-str<strong>and</strong>ed DNA target, these<br />
dyes increase 10- to 20-fold in fluorescence. <strong>Real</strong>-<strong>time</strong> <strong>PCR</strong><br />
takes advantage of this dye property, detecting double the dye<br />
fluorescence with each successive cycle in the exponential<br />
phase of amplification.<br />
These double-str<strong>and</strong>ed DNA-binding dyes are not discriminatory<br />
fluorophores, which means they will bind to any nonspecific<br />
product that is present in the reaction. For this reason, a useful tool<br />
called a “melting curve” is added following the last <strong>PCR</strong> program<br />
step of a SYBR assay. The melting curve comm<strong>and</strong> directs the<br />
thermal block of the real-<strong>time</strong> device to slowly <strong>and</strong> gradually ramp<br />
temperature upward to 95 ºC, taking fluorescent measurements<br />
across <strong>time</strong> <strong>and</strong> temperature. Because DNA hybrids melt, or<br />
“denature,” at varying temperatures based on both varying<br />
sequence <strong>and</strong> length of product—<strong>and</strong> because a release in SYBR<br />
Green fluorescence corresponds to the exact point/temperature<br />
at which a distinct hybrid population’s str<strong>and</strong>s separate, or “melt”—<br />
a real-<strong>time</strong> melting curve shows distinct drops in fluorescence at<br />
each amplicon population’s melting temperatures (Fig. 2). In the<br />
event that a single, nonspecific hybrid population such as a primerdimer<br />
forms during a reaction, two distinct drops in fluorescence<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Unlike the “endpoint measurement” of <strong>PCR</strong> products, real-<strong>time</strong><br />
<strong>PCR</strong> also provides immediate information about the kinetics of the<br />
<strong>PCR</strong> while the fluorescence is plotted out, one cycle to the next,<br />
in “real-<strong>time</strong>.”<br />
Good experimental design can further enable researchers to<br />
acquire a wealth of additional information—down to a detailed<br />
assessment of amplification efficiencies—from one sample to the<br />
next. Clearly, adding fluorescence <strong>and</strong> a highly sensitive optical<br />
detection device to <strong>PCR</strong> opens a world of new possibilities.<br />
at different temperature points of a melting curve analysis can<br />
easily confirm this event. A single, distinct drop in fluorescence at<br />
a single temperature, on the other h<strong>and</strong>, indicates a homogenous<br />
population of highly specific amplified products.<br />
The DNA-binding dye’s ability to bind with any double-str<strong>and</strong>ed<br />
DNA may be interpreted as a disadvantage of lower specificity;<br />
but alternatively, this presents an advantage—you can distinguish<br />
between two hybrid populations with small mutational differences<br />
through the companion tool of melting curves.<br />
This lack of discrimination by DNA-binding dyes is actually a true<br />
advantage in laboratories that wish to look at many different gene<br />
sequences on a routine basis—no oligo design beyond that of the<br />
primer is required, <strong>and</strong> the cost is much less; only primer annealing<br />
needs to be optimized, making it not only a cost-effective chemistry<br />
for real-<strong>time</strong> <strong>PCR</strong>, but also a flexible <strong>and</strong> highly convenient one.<br />
‡ Fig. 2: Melting curve analysis<br />
Raw data shows a dramatic drop in fluorescence when DNA is<br />
melted (denatured); the fact that the drop is seen in several samples<br />
at the same <strong>time</strong> indicates the presence of a single product, i.e., a<br />
highly specific reaction.
Detection chemistries<br />
Probe-based chemistries<br />
Hydrolysis probes, commonly called TaqMan ® probes, are<br />
so-named because they enlist the 5'-endonuclease activity of<br />
Taq polymerase to cleave a reporter dye from their 5'-probe-<br />
terminus. This is significant due to the design of a TaqMan probe,<br />
which not only contains a fluorescent dye terminus but also a<br />
quencher dye at its 3' end. When free in solution or bound to a<br />
complementary sequence of template, each probe molecule’s<br />
quencher disables the nearby reporter dye’s ability to emit<br />
detectable fluorescence. This interaction is termed fluorescence<br />
resonance energy transfer, or “FRET,” because reporter<br />
fluorescence is absorbed by a quencher.<br />
TaqMan probes hybridize in the region between the primers so<br />
that Taq polymerase can activate the reporter during the elongation<br />
step of a <strong>PCR</strong>. As it extends one complement str<strong>and</strong>, Taq 5'-3'-<br />
exonuclease activity releases exactly one reporter dye molecule<br />
that freely emits fluorescence without FRET absorption. Thus, the<br />
correlation between one detected reporter molecule <strong>and</strong> one new<br />
<strong>PCR</strong> product is made.<br />
Pros<br />
‡ High specificity<br />
‡ Duplexing <strong>and</strong> multiplexing<br />
are possible—different reporter<br />
dyes can be selected for<br />
different target sequences,<br />
thus enabling multiple probes<br />
to seek <strong>and</strong> amplify multiple<br />
targets in a single tube<br />
Cons<br />
‡ Require optimization of<br />
probe sequence<br />
‡ Short amplicons,<br />
less sensitive to sample<br />
degradation—e.g., formalin<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
Info<br />
fixed <strong>and</strong> embedded material<br />
‡ Added cost of probe in<br />
addition to primers<br />
Double-str<strong>and</strong>ed DNA-binding dyes, such as SYBR Green, are<br />
an often-used format for optimizing TaqMan assays, as they allow<br />
primers to be optimized before the addition of a probe.<br />
Dye Excitation maximum (nm) Emission maximum (nm)<br />
FAM 494 518<br />
TET 521 538<br />
JOE 520 548<br />
VIC 538 552<br />
Yakima Yellow 526 552<br />
HEX 535 553<br />
NED 546 575<br />
Cy ® 3 552 570<br />
TAMRA 560 582<br />
ROX 587 607<br />
‡ Table 1: Table of dyes frequently used with Mastercycler ® ep realplex systems, <strong>and</strong> their approximate excitation <strong>and</strong> emission<br />
curve peaks<br />
This added specificity comes at a cost—to both the price of probe<br />
synthesis <strong>and</strong> the <strong>time</strong> required for the design <strong>and</strong> optimization<br />
of a specific probe sequence. These chemistries are, therefore,<br />
preferred when a large number of assays are required for a<br />
single, specific target. The advantages <strong>and</strong> disadvantages are<br />
summarized below.<br />
email: info@eppendorf.com • www.eppendorf.com<br />
89<br />
Appendix | <strong>Real</strong>-<strong>time</strong> q<strong>PCR</strong>
<strong>Real</strong>-<strong>time</strong> q<strong>PCR</strong> | Appendix<br />
90<br />
Info<br />
Methods of real-<strong>time</strong> <strong>PCR</strong> quantification<br />
Two primary methods of quantification are routinely used with q<strong>PCR</strong> technology: absolute <strong>and</strong> relative quantification.<br />
Absolute quantification<br />
Absolute quantification is an analysis method to accurately quantify<br />
the exact amount of initial target template in a sample. It does this<br />
through the inclusion of a st<strong>and</strong>ard curve. By offering correlations<br />
between known starting quantities <strong>and</strong> C t values, an unknown<br />
sample’s C t value may be correlated to an associated initial<br />
template quantity (Fig. 3).<br />
This method is measured in units of gene copy number (or pico-<br />
grams or nanograms of DNA) as compared to relative quantification<br />
<strong>and</strong> its ratio value of one target amount compared to another.<br />
Absolute quantification assumes that all st<strong>and</strong>ards <strong>and</strong> samples<br />
have equal amplification efficiencies. As a result, this approach can<br />
pose two key challenges: (1) You must ensure that the amplification<br />
efficiencies of the knowns <strong>and</strong> unknowns are nearly equivalent, <strong>and</strong><br />
(2) the concentration of the serial dilutions should be within<br />
the range of the unknown(s).<br />
The optimization of appropriate controls for reliable absolute<br />
quantification is no small task, <strong>and</strong> in the event RNA is the<br />
initial template it must take into account efficiencies of reverse<br />
transcription as well.<br />
‡ Fig. 3: Absolute quantification<br />
A set of known st<strong>and</strong>ards is run in a q<strong>PCR</strong> reaction, <strong>and</strong> the C ts are<br />
plotted against the known quantities of starting materials; once the<br />
unknown’s C t value is obtained <strong>and</strong> compared, this graph can then<br />
be used to determine its starting quantity.<br />
Relative quantification<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
Relative quantification compares the C t value of one target gene<br />
to another—for example, an internal control or reference gene<br />
(some<strong>time</strong>s called a “housekeeping gene”)—in a single sample.<br />
When RNA is the template, this provides some normalization<br />
effects against variables such as RNA integrity <strong>and</strong> reverse<br />
transcription efficiencies.<br />
In addition to establishing the comparison of a gene of interest<br />
(GOI) to an internal control or reference for that single sample, the<br />
relative quantification method also compares a GOI to the control/<br />
reference gene for some other control sample, called a “calibrator”<br />
(∆∆C t method). The resulting unit of measure is an X-fold change<br />
in gene expression levels; <strong>and</strong> due to the inherent normalization<br />
of the ∆∆C t method, variations in <strong>PCR</strong> efficiency are somewhat<br />
accounted for between samples, which makes it a reliable tool<br />
for gene expression analysis (Fig. 4).<br />
‡ Fig. 4: Relative quantification<br />
This method compares the differences in expression between a<br />
GOI <strong>and</strong> a housekeeping gene of a calibrator control; the ∆∆C t<br />
value shown represents an X-fold change in gene expression.
Methods of primer <strong>and</strong> probe validation<br />
It is essential to validate both the primers <strong>and</strong> probe. For TaqMan ®<br />
probe-based systems, the general rule states that amplicons of<br />
< 150 base pairs (ideally < 100) with a primer melting temperature<br />
(Tm) of ~60 °C <strong>and</strong> a probe Tm between 68 °C <strong>and</strong> 70 °C should be<br />
universally acceptable. The idea is to have the probe anneal first <strong>and</strong><br />
saturate all targets before the primers bind <strong>and</strong> start to extend—this<br />
ensures that all nascent DNA will be quantified.<br />
Probes should not contain multiple repeats, <strong>and</strong> they should avoid<br />
high “G” nucleotide content. Guanine should likewise be excluded<br />
from the 5'-probe terminus, as it has been shown that guanosine<br />
quenches an adjacent fluorophore. Finally, the 3' end of forward<br />
primer should be, optimally, 5 bases from the 5' end of the probe<br />
(within 10 bases is acceptable). Several quencher dyes exist to pair<br />
with reporter dyes.<br />
Primer optimization may be done with conventional <strong>PCR</strong> for those<br />
new to real-<strong>time</strong> <strong>PCR</strong>, <strong>and</strong> results may be analyzed on an agarose<br />
gel or non-denaturing polyacrylamide gel (polyacrylamide is more<br />
sensitive in picking up primer-dimers). The criteria for good primer<br />
performance are as follows:<br />
1. No primer-dimers in the negative controls.<br />
2. Little or no mispriming that would result in mismatched<br />
amplicons—the Impulse <strong>PCR</strong> function of Mastercycler ® ep<br />
realplex’s silver block is a useful feature that avoids these effects<br />
(more on Impulse <strong>PCR</strong> in the <strong>PCR</strong> product highlights section of<br />
this catalog, page 36).<br />
Optimization of forward <strong>and</strong> reverse primer concentrations<br />
It is difficult to design a primer pair with identical melting<br />
temperatures, even though theoretical values may match.<br />
Adjustments to either the annealing temperature or Mg2+ concentration will have a limited affect on improving the<br />
performance of Tm-disassociated primers. If primer pairs fail to<br />
meet the criteria for good performance, a primer matrix across<br />
a span of concentrations may be tested (see “High-speed, real<strong>time</strong><br />
<strong>PCR</strong> assay design for realplex silver block models” in the<br />
<strong>PCR</strong> product highlights section of this catalog, page 38).<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
Info<br />
Primer <strong>and</strong> probe validation: general strategy for new<br />
q<strong>PCR</strong> assay development<br />
Every assay needs to be reoptimized when subject to any new<br />
condition or variable. Recommended optimization includes the<br />
quantification of st<strong>and</strong>ard curve series with high-qualified primers<br />
so that <strong>PCR</strong> efficiency <strong>and</strong> sensitivity may be easily measured.<br />
SYBR Green ® I is a flexible <strong>and</strong> inexpensive option for the<br />
determination of primer quality across a range of tested annealing<br />
temperatures. Mastercycler ep realplex’s gradient option* eases this<br />
optimization effort (see “Gradient function: a highly useful tool for<br />
optimizing real-<strong>time</strong> <strong>PCR</strong>” in the <strong>PCR</strong> product highlights section of<br />
this catalog, page 34).<br />
The issue of specificity is answered by the implementation of a<br />
SYBR melting curve analysis, which provides an additional, valuable<br />
perspective in the primer <strong>and</strong> basic assay optimization effort (Fig. 5).<br />
*U.S. Pat. 6,767,512<br />
90<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
- dI / dT (%) 100<br />
20<br />
10<br />
0<br />
-10<br />
6263<br />
64 6566<br />
6768<br />
6970<br />
7172<br />
7374<br />
7576<br />
7778<br />
7980<br />
8182<br />
8384<br />
8586<br />
8788<br />
8990<br />
9192<br />
9394<br />
95<br />
Temperature [°C]<br />
Threshold: ‡ Fig. 33% 5: Melting curve analysis<br />
Note that in this negative (inverted) first derivative the inflection<br />
point consists of a single peak, indicating a highly specific<br />
<strong>PCR</strong> reaction.<br />
email: info@eppendorf.com • www.eppendorf.com<br />
91<br />
Appendix | <strong>Real</strong>-<strong>time</strong> q<strong>PCR</strong>
<strong>Real</strong>-<strong>time</strong> q<strong>PCR</strong> | Appendix<br />
92<br />
Info<br />
References<br />
1. Hartling I, Weisner RJ. Quantitation of transcript-to-transcript<br />
ratios as a measure of gene expression using RT-<strong>PCR</strong>.<br />
BioTechniques. 1997;23:450-455.<br />
2. Wittwer CT, Herrmann MG, Moss AA, Rasmussen RP.<br />
Continuous fluorescence monitoring of rapid cycle DNA<br />
amplification. BioTechniques. 1997;22:130-138.<br />
3. Rire KM, Rasmussen RP, Wittwer CT. Product differentiation<br />
by analysis of DNA melting curves during the polymerase chain<br />
reaction. Anal. Biochem. 1997;245:154-160.<br />
Resources <strong>and</strong> useful websites<br />
Oligonucleotide <strong>and</strong> assay design resources<br />
Gene Quantification Web page, edited by Michael W. Pfaffl,<br />
contains a host of information relating to q<strong>PCR</strong>:<br />
http://www.gene-quantification.info/<br />
TATAA Biocenter: http://www.tataa.com<br />
GeNorm: http://medgen.ugent.be/~jvdesomp/genorm<br />
Primer 3 Homepage:<br />
http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi<br />
Primer quest Homepage:<br />
http://www.idtdna.com/Scitools/Applications/Primerquest/<br />
Zucker Mfold: www.bioinfo.rpi.edu/~zukerm/<br />
IDT Oligoanalyzer:<br />
http://www.idtdna.com/analyzer/Applications/OligoAnalyzer/<br />
OLIGO Primer Analysis Software: http://www.oligo.net/<br />
Premier Biosoft Beacon Designer:<br />
http://www.premierbiosoft.com/molecular_beacons/<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
4. Gibson UE, Heid CA, Williams PM. A novel method for real <strong>time</strong><br />
quantitative RT-<strong>PCR</strong>. Genome Res. 1996;6:995-1001.<br />
5. Tichopad A, Dilger M, Schwartz G, Pfaffl MW. St<strong>and</strong>ardized<br />
determination of real-<strong>time</strong> <strong>PCR</strong> efficiency from a single reaction<br />
set-up. Nucleic Acids Res. 2003;31:122.<br />
6. Marion JH, Cook P, Miller KS. Accurate <strong>and</strong> statistically verified<br />
quantification of relative mRNA abundances using SYBR Green I<br />
<strong>and</strong> real <strong>time</strong> RT-<strong>PCR</strong>. J. Immunol. Methods 2003;283:291-306.<br />
<strong>Real</strong>-<strong>time</strong> <strong>PCR</strong> primer <strong>and</strong> probe databases<br />
http://medgen.ugent.be/rtprimerdb/<br />
The Primer Bank database, hosted by Harvard University,<br />
contains user-submitted primer sequences for several mouse<br />
<strong>and</strong> human genes:<br />
http://pga.mgh.harvard.edu/primerbank/index.html<br />
The Quantitative <strong>PCR</strong> Primer Database (QPD), maintained by the<br />
National Cancer Institute, contains primer <strong>and</strong> probe sequences for<br />
mouse <strong>and</strong> human genes collected from articles cited in PubMed:<br />
http://web.ncifcrf.gov/rtp/gel/primerdb<br />
Discussion groups<br />
q<strong>PCR</strong> list server: http://groups.yahoo.com/group/qpcrlistserver/
Calculating primer quantity<br />
Conversion to absolute quantity (in pmol)<br />
Primer in pmol =<br />
Example: 0.1 µg of 20 oligomer:<br />
0.1 x 1,000,000<br />
20 x 327<br />
Weight in µg x 1,000,000<br />
Length x 327<br />
= 15.3 pmol primer<br />
Calculating the molar concentration of the primer<br />
Micromolar concentration of primer = pmol/µl<br />
Example 1 Example 2<br />
20 pmol of primer in 100 µl <strong>PCR</strong> mixture = 0.20 micromolar (µM) Primer is 24 nucleotides in length <strong>and</strong> dissolved in 0.1 ml of water<br />
A 10 µl aliquot is diluted to 1.0 ml for A 260 measurement: A 260 = 0.76.<br />
The stock solution has an absorbance at 260 nm (A 260) of 76.<br />
The stock solution (0.1 ml) contains 2.6 A 260 units.<br />
The base composition of the primer is:<br />
A = 6<br />
C = 6<br />
G = 6<br />
T = 6<br />
The molar extinction coefficient at 260 nm for the primer = k (15,200) + l (12,010) + m (7,050) + n (8,400) where:<br />
k = number of A’s<br />
m = number of G’s<br />
l = number of C’s<br />
n = number of T’s<br />
The molar extinction of the <strong>PCR</strong> primer = 6 (15,200) + 6 (12,010) + 6 (7,050) + 6 (8,400) = 255,960 e<br />
The molar concentration of the <strong>PCR</strong> primer stock solution is:<br />
Conversion to weight (in µg)<br />
Weight in µg =<br />
Example: 10 pmol of 25 oligomer:<br />
10 x 25 x 327<br />
76<br />
255,960<br />
1,000,000<br />
= 297 micromolar<br />
pmol x Length x 327<br />
1,000,000<br />
= 0.081 µg primer<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
Info<br />
email: info@eppendorf.com • www.eppendorf.com<br />
93<br />
Appendix | Practical Information
Practical Information | Appendix<br />
94<br />
Info<br />
<strong>PCR</strong> calculator<br />
Optimizing your <strong>PCR</strong> reaction is critical in order to obtain good data. To better assist in this process we offer an online<br />
<strong>PCR</strong> calculator to help you set up various reactions including singleplex <strong>and</strong> multiplex reactions. To access this feature please<br />
visit www.eppendorfna.com/pcrcalculator.<br />
What is the <strong>PCR</strong> calculator?<br />
The composition of every <strong>PCR</strong> preparation has to be calculated<br />
according to the desired concentration of the individual<br />
components (with regard to the volume to be used <strong>and</strong> the<br />
prepared original solutions) as well as the number of samples. Even<br />
Application Calculation template<br />
if the individual components <strong>and</strong> the volume remain the same, the<br />
number of samples may change. The <strong>PCR</strong> calculator calculates the<br />
composition of the MasterMix for your <strong>PCR</strong> preparation.<br />
Conventional <strong>PCR</strong> with a ready-to-use <strong>PCR</strong>-Mix SYBR Assay or Conventional <strong>PCR</strong><br />
Conventional <strong>PCR</strong> with a <strong>PCR</strong>-Mix,<br />
to which further components (up to 4) are added<br />
Conventional multiplex <strong>PCR</strong> (max. 3 targets)<br />
with a ready-to-use <strong>PCR</strong>-Mix<br />
SYBR Green real-<strong>time</strong> <strong>PCR</strong> with a ready-to-use <strong>PCR</strong>-Mix,<br />
which already includes SYBR Green<br />
SYBR Green real-<strong>time</strong> <strong>PCR</strong> with a ready-to-use <strong>PCR</strong>-Mix,<br />
SYBR Green is added separately<br />
SYBR Green real-<strong>time</strong> <strong>PCR</strong> with a <strong>PCR</strong>-Mix, to which further<br />
components (up to 4) are added<br />
Additional Components<br />
Additional Components<br />
The primer couple 2 <strong>and</strong> 3 is recorded as<br />
“Additional Component”<br />
SYBR Assay or Conventional <strong>PCR</strong><br />
SYBR Assay (SYBR not included)<br />
Additional Components<br />
Singleplex, probe-based real-<strong>time</strong> <strong>PCR</strong> with a ready-to-use <strong>PCR</strong> Mix Probe Assay<br />
Singleplex, probe-based real-<strong>time</strong> <strong>PCR</strong> with a <strong>PCR</strong>-Mix,<br />
to which further components (up to 3) are added<br />
Multiplex, probe-based real-<strong>time</strong> <strong>PCR</strong> (max. 3 targets)<br />
with a ready-to-use <strong>PCR</strong>-Mix<br />
How does the <strong>PCR</strong> calculator work?<br />
Our <strong>PCR</strong> calculator facilitates the calculation of a <strong>PCR</strong> preparation.<br />
You can choose different calculation templates for your MasterMix ®<br />
depending on your application. You just need to enter the present<br />
values <strong>and</strong> the calculation of your preparation will be made<br />
automatically. In order to document the calculation you can print<br />
out the calculation sheet <strong>and</strong> add it to your documents.<br />
The calculation basis for the calculator is represented by the<br />
following formula:<br />
C<br />
C<br />
final<br />
initial<br />
X V =<br />
final Vinitial<br />
C final = Final concentration in the <strong>PCR</strong> reaction<br />
C initial = Concentration of the stock solution<br />
V final = Total volume of all <strong>PCR</strong> reactions<br />
V initial = Required volume for the MasterMix<br />
Additional Components<br />
The probe is recorded as “Additional Component”<br />
Multiplex Assay<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
The calculator calculates the MasterMix preparation including<br />
10% excess in order to have enough MasterMix available. The<br />
MasterMix is calculated for the total volume of the <strong>PCR</strong> preparation,<br />
the Template being added separately to each <strong>PCR</strong> reaction <strong>and</strong><br />
not being considered within the MasterMix calculation. If you want<br />
to use a constant volume of Template for your <strong>PCR</strong> preparation<br />
<strong>and</strong> add it to the MasterMix, this can be calculated by using the<br />
calculation template “Additional Components”. The same applies to<br />
the separate addition of dNTPs, magnesium etc.<br />
In the pure MasterMix, the concentration of the individual<br />
components is higher than the subsequent <strong>PCR</strong> preparation, as<br />
the addition of the Template is a dilution. The <strong>PCR</strong> preparation<br />
should be construed so as to preferably mix the same parts by<br />
volume of Template <strong>and</strong> MasterMix. The effect of pipetting errors<br />
can therefore be maintained as small as possible. The smaller the<br />
volume of the Templates to be pipetted, the greater the effect of<br />
pipetting accuracies on the result.<br />
MasterMix <strong>PCR</strong><br />
Reaction<br />
volume<br />
Template
<strong>PCR</strong> calculator<br />
Example:<br />
9 different samples as well as a negative control sample (9+1=10)<br />
shall be examined with a ready-to-use <strong>PCR</strong>-Mix in a singleplex,<br />
probe-based real-<strong>time</strong> <strong>PCR</strong> preparation. Each sample (the negative<br />
control sample, too) is prepared in triplicate (10*3=30). For the<br />
preparation of the MasterMix a 10% reserve is included (30+3=33).<br />
This results in a total number of 33 preparations.<br />
30 Reactions (rxn) of 20 µl + 10% Reserve = 33 rxn =660 µl V final<br />
10 µl MasterMix + 10 µl Template = 20 µl Reaction volume per reaction (rxn)<br />
33 Reactions (rxn) of 10 µl MasterMix = 330 µl MasterMix<br />
C initial C final V initial<br />
Each preparation consists of 50% MasterMix <strong>and</strong> 50% Template<br />
Info<br />
solution, which will be added to each reaction later. Consequently,<br />
the total volume per preparation is 20µl (10µl Template solution<br />
plus 10µl MasterMix). This results in a total volume of 660 µl for the<br />
entire reaction preparation <strong>and</strong> 330 µl for the MasterMix.<br />
Value must be entered Value must be entered Value is calculated<br />
F-primer 100 µM 0.9 µM 5.94 µl (0.9/100*660)<br />
R-primer 100 µM 0.9 µM 5.94 µl (0.9/100*660)<br />
Probe 50 µM 0.2 µM 2.64 µl (0.2/50*660)<br />
<strong>PCR</strong>-Mix 2.5x 1.0x 264.0 µl (1/2.5*660)<br />
Water – – 51.48 µl (330-264-5.94-5.94-2.64)<br />
Volume MasterMix 330.0 µl<br />
Example calculation:<br />
<strong>PCR</strong> reaction set-up calculator Probe q<strong>PCR</strong> Assay<br />
Component Conc. Initial (µM or x) Conc. Final (µM or x) Volume Initial (µl)<br />
F-Primer (µM) 100 0.9 5.94<br />
R-Primer (µM) 100 0.9 5.94<br />
Probe (µM) 50 0.2 2.64<br />
<strong>PCR</strong>-Mic (x fold conc.) 2.5 1 264<br />
Water 51.48<br />
Total Volume MasterMix (µl) 330<br />
Total Volume MasterMix per reaction (µl) 10<br />
Total Volume Template per reaction (µl) 10<br />
Total Volume per reaction (µl) 20<br />
Number of samples 10<br />
Number of replicates 3<br />
Number of total samples 30<br />
Recommended pipetting reserve (10%), e.g. 3 3<br />
Number of total samples + pipetting reserve 33<br />
Total reaction volume (µl) 660<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
95<br />
Appendix | Practical Information
Practical Information | Appendix<br />
96<br />
Info<br />
Abbreviations, symbols <strong>and</strong> conversion factors<br />
Metric prefixes<br />
E = exa = 10 18<br />
P = peta = 10 15<br />
T = tera = 10 12<br />
G = giga = 10 9<br />
M = mega = 10 6<br />
k = kilo = 10 3<br />
h = hecto = 10 2<br />
da = deca = 10 1<br />
d = deci = 10 -1<br />
c = centi = 10 -2<br />
m = milli = 10 -3<br />
µ = micro = 10 -6<br />
n = nano = 10 -9<br />
p = pico = 10 -12<br />
f = femto = 10 -15<br />
a = atto = 10 -18<br />
z = zepto = 10 -21<br />
Tris-HCl buffer, pH values<br />
Nucleic acid conversions<br />
Conversion of weight to absolute quantity (mol)<br />
1 µg of 1,000 bp DNA = 1.52 pmol = 9.1 x 10 11 molecules<br />
1 µg of pUC18/19 DNA (2,686 bp) = 0.57 pmol = 3.4 x 10 11 molecules<br />
1 µg of pBR322 DNA (4,361 bp) = 0.35 pmol = 2.1 x 10 11 molecules<br />
1 µg of M13mp18/19 DNA (7,250 bp) = 0.21 pmol = 1.3 x 10 11 molecules<br />
1 µg of l-DNA (48,502 bp) = 0.03 pmol = 1.8 x 10 10 molecules<br />
Conversion of absolute quantity (mol) to weight<br />
1 pmol of 1,000 bp DNA = 0.66 µg<br />
1 pmol of pUC18/19 DNA (2,686 bp) = 1.77 µg<br />
1 pmol of pBR322 DNA (4,361 bp) = 2.88 µg<br />
1 pmol of M13mp18/19 DNA (7,250 bp) = 4.78 µg<br />
1 pmol of l-DNA (48,502 bp) = 32.01 µg<br />
Common abbreviations<br />
ds double-str<strong>and</strong>ed (as in dsDNA)<br />
ss single-str<strong>and</strong>ed (as in ssDNA)<br />
bp base pair<br />
kb kilobase: 1,000 bases or base pairs, as appropriate<br />
nt nucleotides (base)<br />
Mb megabase: 1,000,000 bp<br />
Da Dalton, the unit of molecular mass;<br />
kDa = 1,000 Da, MDa = 1,000,000 Da<br />
MW molecular weight (g/mol)<br />
M Molar or molarity, moles of solute<br />
per liter of solution (mol/L)<br />
mol Mole, absolute amount of a substance<br />
(1 mol = 6.023 x 10 23 , Avogadro number)<br />
l wavelength<br />
l max<br />
wavelength at the absorption maximum<br />
5 ºC 7.76 7.89 7.97 8.07 8.18 8.26 8.37 8.48 8.58 8.68 8.78 8.88 8.98 9.09 9.18 9.28<br />
25 ºC 7.20 7.30 7.40 7.50 7.60 7.70 7.80 7.90 8.00 8.10 8.20 8.30 8.40 8.50 8.60 8.70<br />
37 ºC 6.91 7.02 7.12 7.22 7.30 7.40 7.52 7.62 7.71 7.80 7.91 8.01 8.10 8.22 8.31 8.42<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.
Abbreviations, symbols <strong>and</strong> conversion factors<br />
Molecular weight of DNA fragments<br />
500 bp dsDNA = 325,000 Da<br />
500 nt (nucleotide) ssDNA = 162,500 Da<br />
1 kb dsDNA = 660,000 Da<br />
1 kb ssDNA = 330,000 Da<br />
1 kb ssRNA = 340,000 Da<br />
1 MDa dsDNA = 1.52 kb<br />
Average molecular weight of dNMP = 325 Da<br />
Average molecular weight of<br />
DNA base pair<br />
Protein conversions<br />
Conversion of proteins to DNA length<br />
= 650 Da<br />
Molecular weights for nucleotides<br />
Compound Molecular weight (in Dalton)<br />
ATP 507.2<br />
CTP 483.2<br />
GTP 523.2<br />
UTP 484.2<br />
dATP 491.2<br />
dCTP 467.2<br />
dGTP 507.2<br />
dTTP 482.2<br />
AMP 347.2<br />
CMP 323.2<br />
GMP 363.2<br />
UMP 324.2<br />
dAMP 312.2<br />
dCMP 288.2<br />
dGMP 328.2<br />
dTMP 303.2<br />
Protein with a molecular weight of 10,000 = 270 bp DNA<br />
Protein with a molecular weight of 30,000 = 810 bp DNA<br />
Protein with a molecular weight of 37,000 (corresponds to 333 amino acids) = 1,000 bp DNA<br />
Protein with a molecular weight of 50,000 = 1.35 kb DNA<br />
Protein with a molecular weight of 100,000 = 2.7 kb DNA<br />
Conversion of absolute quantity (mol) to weight<br />
100 pmoles of 100,000 Da protein = 10 µg<br />
100 pmoles of 50,000 Da protein = 5 µg<br />
100 pmoles of 10,000 Da protein = 1 µg<br />
DNA content of various organisms<br />
Organism DNA content (in bp, haploid genome)<br />
Escherichia coli 4.2 x 10 6<br />
Arabidopsis thaliana 4.7 x 10 6<br />
Saccharomyces cerevisiae 1.4 x 10 7<br />
Drosophila melanogaster 1.4 x 10 8<br />
Homo sapiens 3.3 x 10 9<br />
Triticum aestivum (hexaploid wheat) 1.7 x 10 10<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
Info<br />
email: info@eppendorf.com • www.eppendorf.com<br />
97<br />
Appendix | Practical Information
Practical Information | Appendix<br />
98<br />
Info<br />
Genetic code <strong>and</strong> amino acid properties<br />
Genetic code<br />
1 st Codon position<br />
2 nd Codon position<br />
U C A G<br />
U UUU Phe UCU Ser UAU Tyr UGU Cys U<br />
UUC UCC UAC UGC<br />
UUA Leu UCA UAA Stop UGA Stop<br />
UUG UCG U<strong>AG</strong> Stop UGG Trp<br />
C CUU Leu CCU Pro CAU His CGU Arg U<br />
CUC CCC CAC CGC<br />
CUA CCA CAA Gln CGA<br />
CUG CCG C<strong>AG</strong> CGG<br />
A AUU Ile ACU Thr AAU Asn <strong>AG</strong>U Ser U<br />
AUC ACC AAC <strong>AG</strong>C<br />
AUA ACA AAA Lys <strong>AG</strong>A Arg<br />
AUG Met, Start ACG A<strong>AG</strong> <strong>AG</strong>G<br />
G GUU Val GCU Ala GAU Asp GGU Gly U<br />
GUC GCC GAC GGC<br />
GUA GCA GAA Glu GGA<br />
GUG GCG G<strong>AG</strong> GGG<br />
Nomenclature <strong>and</strong> properties of amino acids<br />
C<br />
A<br />
G<br />
C<br />
A<br />
G<br />
C<br />
A<br />
G<br />
C<br />
A<br />
G<br />
3 rd Codon position<br />
Termination codons:<br />
UAA: ochre<br />
U<strong>AG</strong>: amber<br />
UGA: opal<br />
Amino acid 3-letter symbol 1-letter symbol Major properties of side chains<br />
Alanine Ala A Aliphatic<br />
Arginine Arg R Basic group<br />
Asparagine Asn N Amide group<br />
Aspartic acid Asp D Acidic group<br />
Cysteine Cys C Sulfur-containing<br />
Glutamic acid Glu E Acidic group<br />
Glutamine Gln Q Amide group<br />
Glycine Gly G No side chain<br />
Histidine His H Imidazole group<br />
Isoleucine Ile I Aliphatic<br />
Leucine Leu L Aliphatic<br />
Lysine Lys K Basic group<br />
Methionine Met M Sulfur-containing<br />
Phenylalanine Phe F Aromatic group<br />
Proline Pro P Aliphatic<br />
Serine Ser S Hydroxyl group<br />
Threonine Thr T Hydroxyl group<br />
Tryptophan Trp W Aromatic group<br />
Tyrosine Tyr Y Aromatic group<br />
Valine Val V Aliphatic<br />
Product appearance, specifications, <strong>and</strong>/or prices are subject to change without notice.<br />
In yeast mitochondria, the AUA<br />
<strong>and</strong> UGA codons are used<br />
for Met <strong>and</strong> Trp, not for Ile <strong>and</strong><br />
Stop as normally.<br />
Start codon:<br />
AUG: Methionine
Your gateway to everything <strong>Eppendorf</strong>:<br />
Access many useful website features<br />
when you register with my<strong>Eppendorf</strong><br />
‡ Customer Care Center<br />
‡ Online product registration<br />
‡ ep-points customer<br />
rewards program<br />
‡ <strong>Eppendorf</strong> eShop<br />
For Application Support<br />
Email:<br />
arts@eppendorf.com<br />
Hotline:<br />
800-645-3050 ext. 2258<br />
Local Sales Support<br />
Use our Sales Rep Locator to get in touch<br />
with your local ARTS Specialist.<br />
<strong>Eppendorf</strong> Awards<br />
We promote science! Learn about the<br />
$25,000 <strong>Eppendorf</strong> & Science Prize at<br />
www.eppendorf.com/awards.<br />
<strong>Eppendorf</strong> ® <strong>and</strong> design, epMotion ® , <strong>Eppendorf</strong> Tubes ® , Mastercycler ® , <strong>and</strong> SteadySlope ® are registered trademarks of<br />
<strong>Eppendorf</strong> <strong>AG</strong>. epBlueis a trademark of <strong>Eppendorf</strong> <strong>AG</strong>. <strong>Eppendorf</strong> ARTS <strong>and</strong> design is a trademark of <strong>Eppendorf</strong><br />
North America. Agilent ® is a registered trademark of Agilent Technologies, Inc. American Express ® is a registered trademark<br />
of The American Express Company. CAL Fluor ® is a registered trademark of Biosearch Technologies, Inc. Cy ® 3 is a registered<br />
trademark of Amersham Biosciences UK Limited. FAM <strong>and</strong> TAMRA are trademarks of Applied Biosystems. Invitrogen ® ,<br />
LUX Primer, PureLink ® <strong>and</strong> Superscript ® are registered trademarks of Invitrogen Corporation. LabChip ® is a registered<br />
trademark of Caliper Life Sciences, Inc. LightCycler ® is a registered trademark of Roche Diagnostics GmbH. MasterCard ®<br />
is a registered trademark of MasterCard International Incorporated. Microsoft ® <strong>and</strong> Windows ® are registered trademarks of<br />
Microsoft Corporation. NucleoSpin ® is a registered trademark of Macherey, Nagel GmbH & Co. Promega ® <strong>and</strong> Promega<br />
Wizard ® are registered trademarks of Promega Corporation. QI<strong>AG</strong>EN ® , Biorobot ® , QiaAmp ® <strong>and</strong> Rneasy ® are registered trademarks<br />
of QI<strong>AG</strong>EN GmbH or its subsidies in the US <strong>and</strong> certain other countries. HotMaster ® , MasterMix ® <strong>and</strong> Perfectprep ® are<br />
registered trademarks of 5 PRIME GmbH. Roche ® is a registered trademark of Hoffmann-La Roche Inc. ROX is a trademark<br />
of Applera Corporation or its subsidies in the US <strong>and</strong> certain other countries. ABI PRISM ® is a registered trademark of Applera<br />
Corporation or its subsidies in the US <strong>and</strong> certain other countries. ABI BigDye is a trademark of Applera Corporation or its<br />
subsidies in the US <strong>and</strong> certain other countries. SmartCycler ® is a registered trademark of Cepheid Corporation. SuperMix ®<br />
is a registered trademark of Gentest Corporation. SYBR ® is a registered trademark of Molecular Probes, Inc. TaqMan ® is a<br />
registered trademark of Roche Molecular Systems, Inc. VISA ® is a registered trademark of IBANCO Ltd.<br />
www.eppendorf.com<br />
Online Support<br />
Visit our online Resource Center to view<br />
<strong>and</strong> download a wealth of information:<br />
‡ Applications<br />
‡ FAQs<br />
‡ Product manuals<br />
‡ Material safety data sheets<br />
‡ Product quality certificates<br />
‡ Product literature<br />
Service<br />
Learn about our calibration, preventive<br />
maintenance <strong>and</strong> repair services—all provided<br />
by manufacturer-direct technicians.<br />
<strong>Eppendorf</strong> Advantage<br />
View our latest product promotions.<br />
Mastercycler ep: Practice of the patented polymerase chain reaction (<strong>PCR</strong>) process requires a license. The <strong>Eppendorf</strong> ®<br />
Thermal Cycler is an Authorized Thermal Cycler <strong>and</strong> may be used with <strong>PCR</strong> licenses available from Applied Biosystems. Its use<br />
with Authorized Reagents also provides a limited <strong>PCR</strong> license in accordance with the label rights accompanying such reagents.<br />
Mastercycler ep realplex: Practice of the patented polymerase chain reaction (<strong>PCR</strong>) process requires a license. The<br />
<strong>Eppendorf</strong> ® Thermal Cycler is an Authorized Thermal Cycler <strong>and</strong> may be used with <strong>PCR</strong> licenses available from Applied<br />
Biosystems. Its use with Authorized Reagents also provides a limited <strong>PCR</strong> license in accordance with the label rights<br />
accompanying such reagents. This is a Licensed <strong>Real</strong>-Time Thermal Cycler under Applera’s United States Patent No.<br />
6,814,934 <strong>and</strong> corresponding claims in non-U.S. counterparts thereof, for use in research <strong>and</strong> for all other applied fields<br />
except human in vitro diagnostics. No right is conveyed expressly, by implication or by estoppel under any other patent claim.<br />
In the U.S.: <strong>Eppendorf</strong> North America 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
email: info@eppendorf.com • www.eppendorf.com<br />
99
Support <strong>and</strong> Services Directory<br />
Contact Information<br />
United States Canada<br />
Business Hours: 8:30 a.m. to 6:00 p.m. EST 8:30 a.m. to 5:00 p.m. EST<br />
Phone: 800-645-3050<br />
516-334-7500<br />
800-263-8715<br />
905-826-5525<br />
Fax: 516-334-7506 905-826-5424<br />
Address: <strong>Eppendorf</strong> North America, Inc.<br />
One Cantiague Road<br />
Westbury, NY 11590-0207<br />
<strong>Eppendorf</strong> Canada Ltd.<br />
2810 Argentia Road, #2<br />
Mississauga, ON L5N 8L2<br />
Website: www.eppendorfna.com www.eppendorf.ca<br />
Email: info@eppendorf.com canada@eppendorf.com<br />
Customer Support: 800-645-3050, ext. 2101<br />
custserv@eppendorf.com<br />
Repair/Service<br />
Support:<br />
Applications<br />
Support:<br />
800-645-3050, ext. 2405<br />
service@eppendorf.com<br />
800-645-3050, ext. 2258<br />
apps@eppendorf.com<br />
800-263-8715, menu option 1<br />
canadacustserv@eppendorf.com<br />
800-263-8715, ext. 3231<br />
canadaservice@eppendorf.com<br />
800-645-3050, ext. 2258 (U.S.)<br />
apps@eppendorf.com<br />
www.eppendorf.com • Email: info@eppendorf.com • Application hotline: 516-515-2258<br />
In the U.S.: <strong>Eppendorf</strong> North America, Inc. 800-645-3050 • In Canada: <strong>Eppendorf</strong> Canada Ltd. 800-263-8715<br />
ENA.C1.0120.B.US-7.0 © 2008 <strong>Eppendorf</strong> <strong>AG</strong>.