The Mitochondrial Free Radical Theory of Aging - Supernova: Pliki
The Mitochondrial Free Radical Theory of Aging - Supernova: Pliki
The Mitochondrial Free Radical Theory of Aging - Supernova: Pliki
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
A Challenge from Textbook Bioenergetics and <strong>Free</strong> <strong>Radical</strong> Chemistry<br />
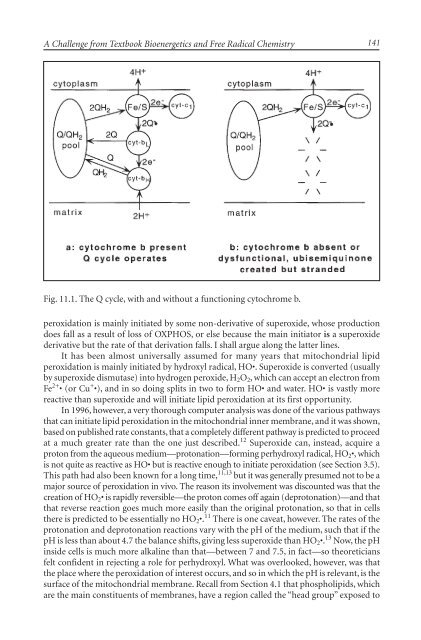
Fig. 11.1. <strong>The</strong> Q cycle, with and without a functioning cytochrome b.<br />
peroxidation is mainly initiated by some non-derivative <strong>of</strong> superoxide, whose production<br />
does fall as a result <strong>of</strong> loss <strong>of</strong> OXPHOS, or else because the main initiator is a superoxide<br />
derivative but the rate <strong>of</strong> that derivation falls. I shall argue along the latter lines.<br />
It has been almost universally assumed for many years that mitochondrial lipid<br />
peroxidation is mainly initiated by hydroxyl radical, HO•. Superoxide is converted (usually<br />
by superoxide dismutase) into hydrogen peroxide, H2O2, which can accept an electron from<br />
Fe 2+ • (or Cu + •), and in so doing splits in two to form HO• and water. HO• is vastly more<br />
reactive than superoxide and will initiate lipid peroxidation at its first opportunity.<br />
In 1996, however, a very thorough computer analysis was done <strong>of</strong> the various pathways<br />
that can initiate lipid peroxidation in the mitochondrial inner membrane, and it was shown,<br />
based on published rate constants, that a completely different pathway is predicted to proceed<br />
at a much greater rate than the one just described. 12 Superoxide can, instead, acquire a<br />
proton from the aqueous medium—protonation—forming perhydroxyl radical, HO2•, which<br />
is not quite as reactive as HO• but is reactive enough to initiate peroxidation (see Section 3.5).<br />
This path had also been known for a long time, 11,13 but it was generally presumed not to be a<br />
major source <strong>of</strong> peroxidation in vivo. <strong>The</strong> reason its involvement was discounted was that the<br />
creation <strong>of</strong> HO2• is rapidly reversible—the proton comes <strong>of</strong>f again (deprotonation)—and that<br />
that reverse reaction goes much more easily than the original protonation, so that in cells<br />
there is predicted to be essentially no HO2•. 11 <strong>The</strong>re is one caveat, however. <strong>The</strong> rates <strong>of</strong> the<br />
protonation and deprotonation reactions vary with the pH <strong>of</strong> the medium, such that if the<br />
pH is less than about 4.7 the balance shifts, giving less superoxide than HO2•. 13 Now, the pH<br />
inside cells is much more alkaline than that—between 7 and 7.5, in fact—so theoreticians<br />
felt confident in rejecting a role for perhydroxyl. What was overlooked, however, was that<br />
the place where the peroxidation <strong>of</strong> interest occurs, and so in which the pH is relevant, is the<br />
surface <strong>of</strong> the mitochondrial membrane. Recall from Section 4.1 that phospholipids, which<br />
are the main constituents <strong>of</strong> membranes, have a region called the “head group” exposed to<br />
141