Macrocyclic Ligands - Web del Profesor
Macrocyclic Ligands - Web del Profesor
Macrocyclic Ligands - Web del Profesor
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
16 MACROCYCLIC LIGANDS<br />
O<br />
S<br />
O<br />
N N<br />
Cu<br />
O O<br />
O<br />
N<br />
Ba<br />
Cu<br />
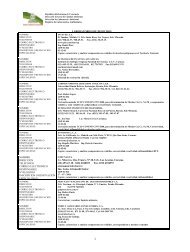
(63)<br />
occurs for the potassium ion, which has a radius of 1.38 ˚A, thus<br />
correlating well with the cavity radius. However, 18-crown-6<br />
forms extremely stable complexes with all of the alkali and<br />
alkaline earth metal ions. Hence, Gokel argues that the data<br />
indicate that the hole size concept is inapplicable, since the<br />
binding constants for sodium, potassium, ammonium, and<br />
calcium ions are the largest for the 18-crown-6 compared to<br />
almost all of the other simple crown ethers. 119 Hancock has<br />
proposed that chelate ring size is the critical factor, and that the<br />
high stabilities observed for the crown ethers with large metal<br />
ions is a result of the presence of five-membered chelate rings.<br />
Thus the high affinity of these macrocycles for the potassium<br />
ion is explained by the fact that potassium is the right size for<br />
the five-membered chelate rings of the crown ethers. 78<br />
O<br />
O<br />
O<br />
(64)<br />
O<br />
O<br />
O<br />
O<br />
O<br />
(65)<br />
O<br />
O<br />
O<br />
N<br />
O<br />
O<br />
S<br />
O<br />
O<br />
O<br />
O<br />
O<br />
(66)<br />
A number of reviews of the structural aspects of crown<br />
ethers can be found. 115–117 These structures vary considerably<br />
in complexity. An example of the flexibility of the crown ethers<br />
can be seen in the variation in the structures as a result of ring<br />
size of three different benzo crowns. When the cavity of the<br />
O<br />
O<br />
O<br />
crown matches the radius of the metal ion, the metal ion can be<br />
readily incorporated in the cavity, such as in the structure of the<br />
rubidium thiocyanate complex with the dibenzo-18-crown-6<br />
(67). In cases where the cavity of the crown is too large to<br />
surround the metal ion snugly, a folded structure can result,<br />
as with the dibenzo-30-crown-10 (68) and the potassium ion.<br />
For very large metal ions incapable of fitting into smaller<br />
macrocyclic cavities, sandwich-type structures can occur, as<br />
in the benzo-15-crown-5 (69) with the potassium ion. 115<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
(67)<br />
O<br />
O<br />
O O O<br />
O O O<br />
O<br />
(68)<br />
O<br />
O O<br />
(69)<br />
Molecular mechanics studies indicate that the lowest energy<br />
conformer of the uncomplexed ligand is not necessarily<br />
that required for complexation, i.e. oxygen donors may be<br />
exodentate as in the thia macrocycles. This means that in order<br />
for complex formation to occur, the ligand must undergo both<br />
reorganization as well as desolvation. A general rule of thumb<br />
with respect to size, however, is that the larger macrocycles<br />
are more flexible and subject to adaptability, while the smaller<br />
macrocycles are more rigid and, in that sense, ‘preorganized’.<br />
Cram has provided an excellent treatise on preorganization. 118<br />
His principle of preorganization is that ‘the more highly<br />
hosts and guests are organized for binding and low solvation<br />
prior to their complexation, the more stable will be their<br />
complexes.’ 118 �G values for a variety of macrocyclic oxygen<br />
donors indicate that the ‘prearranged’ ligands in general bind<br />
O<br />
O<br />
O