NCI National Clinical Trials Network (NCTN) Program Guidelines
NCI National Clinical Trials Network (NCTN) Program Guidelines NCI National Clinical Trials Network (NCTN) Program Guidelines
PART 4: Appendices Section II – Required Tables for New/Competing & Non-Competing Applications Table 5. Summary Accrual by Lead Academic Participating Site Applicant for All Clinical Trials for All Cancers by Trial Phase (Include accrual only over the past 5-6 years) Type Study Accrual Accrual Period (MM/DD/YYYY to MM/DD/YYYY) Accrual by Main Lead Academic Center PILOT Treatment Studies PHASE 1 Tx Studies PHASE 2 Treatment Studies (includes Phase 1/2 Studies) Screened Only Screened & Intervention Total Pts PHASE 3 Treatment Studies (includes Phase 2/3 Studies) Screened Only Screened & Intervention Total Pts All Treatment Studies (Include accrual from all treatment trials for all phases and pilot studies – if trial does not have a screening component, then it should be totaled in the “screened and intervention” component category)* Screened Only Screened & Intervention 5 5 15 50 65 20 100 120 35 160 195 Accrual by Lead Academic Component #1 (e.g. if academic center has a clinic at a different geographic location with a different NCI institution code) 1 1 5 10 15 20 30 50 25 42 67 Accrual by Affiliate(s) if part of application TOTALS: Type Study Accrual Accrual Period (MM/DD/YYYY to MM/DD/YYYY) Per Patient Biospecimen Collection Accrual by Main Lead Academic Center Per Patient Biospecimen Collection by Lead Academic Component #1 (e.g. if academic center has a clinic at a different geographic location with a different NCI institution code) Per Patient Biospecimen Collection by Affiliate(s) if part of application 1 1 5 10 15 20 30 50 25 42 67 7 7 25 70 95 60 160 220 85 244 329 PILOT Treatment Studies PHASE 1 Tx Studies PHASE 2 Treatment Studies (includes Phase 1/2 Studies) Screened Only Screened & Intervention Total PHASE 3 Treatment Studies (includes Phase 2/3 Studies) Screened Only Screened & Intervention Total Page 206 of 241 Total Pts All Treatment Studies (Include accrual from all treatment trials for all phases and pilot studies – if trial does not have a screening component, then it should be totaled in the “screened and intervention” component category)* Screened Only Screened & Intervention Note: This is # Pts with ≥1 specimen(s) (NOT # of specimens) 3 2 15 50 65 20 100 120 35 155 190 Note: This is # Pts with ≥1 specimen(s) (NOT # of specimens) 1 Note: This is # Pts with ≥1 specimen(s) (NOT # of specimens) 1 5 10 15 20 30 50 25 42 67 1 1 5 10 15 20 30 50 25 42 67 *Accrual figures should include eligible & ineligible patients. Screened patients refer to patients who are screened only for studies that have screening as a distinct part of the protocol - patients are consented and screened as part of the trial but may not undergo randomization/intervention because the screening excludes them from that part of the study (i.e., patient’s tumor did not have the required characteristic for treatments if the tumor is tested as part of the study). “Screened Only” and “Screened and Intervention” are mutually exclusive categories of accrual. Pilot studies refer to studies testing feasibility of administration of therapeutic intervention/approach and/or are explicitly determined to be “pilot studies” at the time of NCI/DCTD approval. Biospecimen information is for collections for patients enrolled on these tx trials only. Total
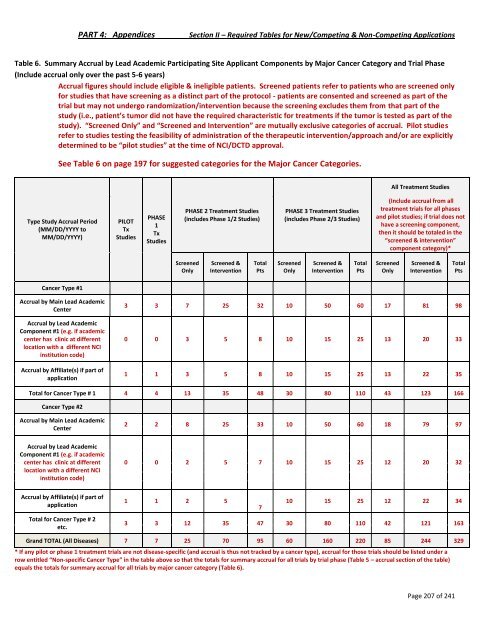
PART 4: Appendices Section II – Required Tables for New/Competing & Non-Competing Applications Table 6. Summary Accrual by Lead Academic Participating Site Applicant Components by Major Cancer Category and Trial Phase (Include accrual only over the past 5-6 years) Accrual figures should include eligible & ineligible patients. Screened patients refer to patients who are screened only for studies that have screening as a distinct part of the protocol - patients are consented and screened as part of the trial but may not undergo randomization/intervention because the screening excludes them from that part of the study (i.e., patient’s tumor did not have the required characteristic for treatments if the tumor is tested as part of the study). “Screened Only” and “Screened and Intervention” are mutually exclusive categories of accrual. Pilot studies refer to studies testing the feasibility of administration of the therapeutic intervention/approach and/or are explicitly determined to be “pilot studies” at the time of NCI/DCTD approval. See Table 6 on page 197 for suggested categories for the Major Cancer Categories. Type Study Accrual Period (MM/DD/YYYY to MM/DD/YYYY) Cancer Type #1 Accrual by Main Lead Academic Center Accrual by Lead Academic Component #1 (e.g. if academic center has clinic at different location with a different NCI institution code) Accrual by Affiliate(s) if part of application PILOT Tx Studies PHASE 1 Tx Studies PHASE 2 Treatment Studies (includes Phase 1/2 Studies) Screened Only Screened & Intervention Total Pts PHASE 3 Treatment Studies (includes Phase 2/3 Studies) Screened Only Screened & Intervention Total Pts All Treatment Studies (Include accrual from all treatment trials for all phases and pilot studies; if trial does not have a screening component, then it should be totaled in the “screened & intervention” component category)* Screened Only Screened & Intervention 3 3 7 25 32 10 50 60 17 81 98 0 0 3 5 8 10 15 25 13 20 33 1 1 3 5 8 10 15 25 13 22 35 Total for Cancer Type # 1 4 4 13 35 48 30 80 110 43 123 166 Cancer Type #2 Accrual by Main Lead Academic Center Accrual by Lead Academic Component #1 (e.g. if academic center has clinic at different location with a different NCI institution code) Accrual by Affiliate(s) if part of application Total for Cancer Type # 2 etc. 2 2 8 25 33 10 50 60 18 79 97 0 0 2 5 7 10 15 25 12 20 32 1 1 2 5 7 Page 207 of 241 Total Pts 10 15 25 12 22 34 3 3 12 35 47 30 80 110 42 121 163 Grand TOTAL (All Diseases) 7 7 25 70 95 60 160 220 85 244 329 * If any pilot or phase 1 treatment trials are not disease-specific (and accrual is thus not tracked by a cancer type), accrual for those trials should be listed under a row entitled “Non-specific Cancer Type” in the table above so that the totals for summary accrual for all trials by trial phase (Table 5 – accrual section of the table) equals the totals for summary accrual for all trials by major cancer category (Table 6).
- Page 155 and 156: PART 2: Submission of New/Competing
- Page 157 and 158: PART 2: Submission of New/Competing
- Page 159 and 160: PART 2: Submission of New/Competing
- Page 161 and 162: PART 2: Submission of New/Competing
- Page 163 and 164: PART 2: Submission of New/Competing
- Page 165 and 166: PART 2: Submission of New/Competing
- Page 167 and 168: PART 2: Submission of New/Competing
- Page 169 and 170: PART 2: Submission of New/Competing
- Page 171 and 172: PART 2: Submission of New/Competing
- Page 173 and 174: PART 2: Submission of New/Competing
- Page 175 and 176: PART 2: Submission of New/Competing
- Page 177 and 178: PART 2: Submission of New/Competing
- Page 179 and 180: PART 2: Submission of New/Competing
- Page 181 and 182: PART 2: Submission of New/Competing
- Page 183 and 184: PART 2: Submission of New/Competing
- Page 185 and 186: PART 2: Submission of New/Competing
- Page 187 and 188: PART 3: Submission of Non-Competing
- Page 189 and 190: TYPE OF STUDY PART 3: Submission No
- Page 191 and 192: PART 3: Submission Non-Competing Ap
- Page 193 and 194: PART 3: Submission Non-Competing Ap
- Page 195 and 196: PART 4: Appendices Section II - Req
- Page 197 and 198: PART 4: Appendices Section II - Req
- Page 199 and 200: PART 4: Appendices Section II - Req
- Page 201 and 202: Participating Site Member Category
- Page 203 and 204: PART 4: Appendices Section II - Req
- Page 205: PART 4: Appendices Section II - Req
- Page 209 and 210: PART 4: Appendices Section II - Req
- Page 211 and 212: PART 4: Appendices Section IV - Cos
- Page 213 and 214: PART 4: Appendices Section IV - Cos
- Page 215 and 216: PART 4: Appendices Section IV - Cos
- Page 217 and 218: PART 4: Appendices Section V - Impo
- Page 219 and 220: PART 4: Appendices Section V - Impo
- Page 221 and 222: PART 4: Appendices Section V - Impo
- Page 223 and 224: Part 4: Appendices - VI. Sample Tab
- Page 225 and 226: Part 4: Appendices - VII. Model for
- Page 227 and 228: Part 4: Appendices - VII. NCTN Prog
- Page 229 and 230: Part 4: Appendices - VII. NCTN Prog
- Page 231 and 232: Part 4: Appendices - VII. NCTN Prog
- Page 233 and 234: Part 4: Appendices - IX. Common Bud
- Page 235 and 236: Part 4: Appendices - IX. Common Bud
- Page 237 and 238: Part 4: Appendices - VII. Accrual I
- Page 239 and 240: Part 4: Summary of Updates to Guide
- Page 241: Part 4: Summary of Updates to Guide
PART 4: Appendices Section II – Required Tables for New/Competing & Non-Competing Applications<br />
Table 6. Summary Accrual by Lead Academic Participating Site Applicant Components by Major Cancer Category and Trial Phase<br />
(Include accrual only over the past 5-6 years)<br />
Accrual figures should include eligible & ineligible patients. Screened patients refer to patients who are screened only<br />
for studies that have screening as a distinct part of the protocol - patients are consented and screened as part of the<br />
trial but may not undergo randomization/intervention because the screening excludes them from that part of the<br />
study (i.e., patient’s tumor did not have the required characteristic for treatments if the tumor is tested as part of the<br />
study). “Screened Only” and “Screened and Intervention” are mutually exclusive categories of accrual. Pilot studies<br />
refer to studies testing the feasibility of administration of the therapeutic intervention/approach and/or are explicitly<br />
determined to be “pilot studies” at the time of <strong>NCI</strong>/DCTD approval.<br />
See Table 6 on page 197 for suggested categories for the Major Cancer Categories.<br />
Type Study Accrual Period<br />
(MM/DD/YYYY to<br />
MM/DD/YYYY)<br />
Cancer Type #1<br />
Accrual by Main Lead Academic<br />
Center<br />
Accrual by Lead Academic<br />
Component #1 (e.g. if academic<br />
center has clinic at different<br />
location with a different <strong>NCI</strong><br />
institution code)<br />
Accrual by Affiliate(s) if part of<br />
application<br />
PILOT<br />
Tx<br />
Studies<br />
PHASE<br />
1<br />
Tx<br />
Studies<br />
PHASE 2 Treatment Studies<br />
(includes Phase 1/2 Studies)<br />
Screened<br />
Only<br />
Screened &<br />
Intervention<br />
Total<br />
Pts<br />
PHASE 3 Treatment Studies<br />
(includes Phase 2/3 Studies)<br />
Screened<br />
Only<br />
Screened &<br />
Intervention<br />
Total<br />
Pts<br />
All Treatment Studies<br />
(Include accrual from all<br />
treatment trials for all phases<br />
and pilot studies; if trial does not<br />
have a screening component,<br />
then it should be totaled in the<br />
“screened & intervention”<br />
component category)*<br />
Screened<br />
Only<br />
Screened &<br />
Intervention<br />
3 3 7 25 32 10 50 60 17 81 98<br />
0 0 3 5 8 10 15 25 13 20 33<br />
1 1 3 5 8 10 15 25 13 22 35<br />
Total for Cancer Type # 1 4 4 13 35 48 30 80 110 43 123 166<br />
Cancer Type #2<br />
Accrual by Main Lead Academic<br />
Center<br />
Accrual by Lead Academic<br />
Component #1 (e.g. if academic<br />
center has clinic at different<br />
location with a different <strong>NCI</strong><br />
institution code)<br />
Accrual by Affiliate(s) if part of<br />
application<br />
Total for Cancer Type # 2<br />
etc.<br />
2 2 8 25 33 10 50 60 18 79 97<br />
0 0 2 5 7 10 15 25 12 20 32<br />
1 1 2 5<br />
7<br />
Page 207 of 241<br />
Total<br />
Pts<br />
10 15 25 12 22 34<br />
3 3 12 35 47 30 80 110 42 121 163<br />
Grand TOTAL (All Diseases) 7 7 25 70 95 60 160 220 85 244 329<br />
* If any pilot or phase 1 treatment trials are not disease-specific (and accrual is thus not tracked by a cancer type), accrual for those trials should be listed under a<br />
row entitled “Non-specific Cancer Type” in the table above so that the totals for summary accrual for all trials by trial phase (Table 5 – accrual section of the table)<br />
equals the totals for summary accrual for all trials by major cancer category (Table 6).