Graphene Sheets from Graphitized Anthracite Coal: Preparation ...
Graphene Sheets from Graphitized Anthracite Coal: Preparation ...
Graphene Sheets from Graphitized Anthracite Coal: Preparation ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Energy & Fuels Article<br />
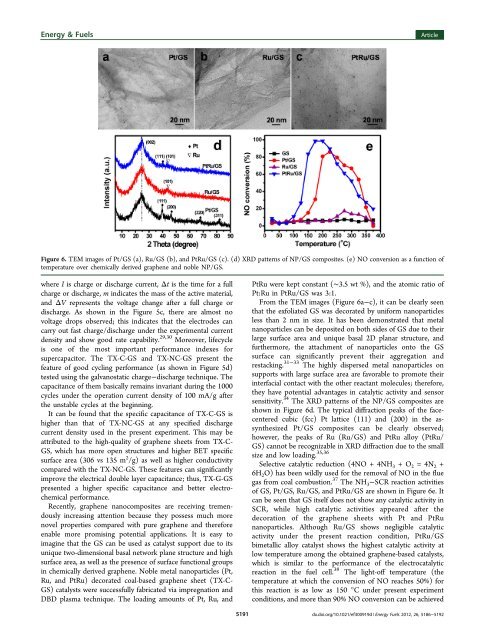
Figure 6. TEM images of Pt/GS (a), Ru/GS (b), and PtRu/GS (c). (d) XRD patterns of NP/GS composites. (e) NO conversion as a function of<br />
temperature over chemically derived graphene and noble NP/GS.<br />
where I is charge or discharge current, Δt is the time for a full<br />
charge or discharge, m indicates the mass of the active material,<br />
and ΔV represents the voltage change after a full charge or<br />
discharge. As shown in the Figure 5c, there are almost no<br />
voltage drops observed; this indicates that the electrodes can<br />
carry out fast charge/discharge under the experimental current<br />
density and show good rate capability. 29,30 Moreover, lifecycle<br />
is one of the most important performance indexes for<br />
supercapacitor. The TX-C-GS and TX-NC-GS present the<br />
feature of good cycling performance (as shown in Figure 5d)<br />
tested using the galvanostatic charge−discharge technique. The<br />
capacitance of them basically remains invariant during the 1000<br />
cycles under the operation current density of 100 mA/g after<br />
the unstable cycles at the beginning.<br />
It can be found that the specific capacitance of TX-C-GS is<br />
higher than that of TX-NC-GS at any specified discharge<br />
current density used in the present experiment. This may be<br />
attributed to the high-quality of graphene sheets <strong>from</strong> TX-C-<br />
GS, which has more open structures and higher BET specific<br />
surface area (306 vs 135 m 2 /g) as well as higher conductivity<br />
compared with the TX-NC-GS. These features can significantly<br />
improve the electrical double layer capacitance; thus, TX-G-GS<br />
presented a higher specific capacitance and better electrochemical<br />
performance.<br />
Recently, graphene nanocomposites are receiving tremendously<br />
increasing attention because they possess much more<br />
novel properties compared with pure graphene and therefore<br />
enable more promising potential applications. It is easy to<br />
imagine that the GS can be used as catalyst support due to its<br />
unique two-dimensional basal network plane structure and high<br />
surface area, as well as the presence of surface functional groups<br />
in chemically derived graphene. Noble metal nanoparticles (Pt,<br />
Ru, and PtRu) decorated coal-based graphene sheet (TX-C-<br />
GS) catalysts were successfully fabricated via impregnation and<br />
DBD plasma technique. The loading amounts of Pt, Ru, and<br />
5191<br />
PtRu were kept constant (∼3.5 wt %), and the atomic ratio of<br />
Pt:Ru in PtRu/GS was 3:1.<br />
From the TEM images (Figure 6a−c), it can be clearly seen<br />
that the exfoliated GS was decorated by uniform nanoparticles<br />
less than 2 nm in size. It has been demonstrated that metal<br />
nanoparticles can be deposited on both sides of GS due to their<br />
large surface area and unique basal 2D planar structure, and<br />
furthermore, the attachment of nanoparticles onto the GS<br />
surface can significantly prevent their aggregation and<br />
restacking. 31−33 The highly dispersed metal nanoparticles on<br />
supports with large surface area are favorable to promote their<br />
interfacial contact with the other reactant molecules; therefore,<br />
they have potential advantages in catalytic activity and sensor<br />
sensitivity. 34 The XRD patterns of the NP/GS composites are<br />
shown in Figure 6d. The typical diffraction peaks of the facecentered<br />
cubic (fcc) Pt lattice (111) and (200) in the assynthesized<br />
Pt/GS composites can be clearly observed;<br />
however, the peaks of Ru (Ru/GS) and PtRu alloy (PtRu/<br />
GS) cannot be recognizable in XRD diffraction due to the small<br />
size and low loading. 35,36<br />
Selective catalytic reduction (4NO + 4NH 3 +O 2 =4N 2 +<br />
6H 2O) has been wildly used for the removal of NO in the flue<br />
gas <strong>from</strong> coal combustion. 37 The NH 3−SCR reaction activities<br />
of GS, Pt/GS, Ru/GS, and PtRu/GS are shown in Figure 6e. It<br />
can be seen that GS itself does not show any catalytic activity in<br />
SCR, while high catalytic activities appeared after the<br />
decoration of the graphene sheets with Pt and PtRu<br />
nanoparticles. Although Ru/GS shows negligible catalytic<br />
activity under the present reaction condition, PtRu/GS<br />
bimetallic alloy catalyst shows the highest catalytic activity at<br />
low temperature among the obtained graphene-based catalysts,<br />
which is similar to the performance of the electrocatalytic<br />
reaction in the fuel cell. 38 The light-off temperature (the<br />
temperature at which the conversion of NO reaches 50%) for<br />
this reaction is as low as 150 °C under present experiment<br />
conditions, and more than 90% NO conversion can be achieved<br />
dx.doi.org/10.1021/ef300919d | Energy Fuels 2012, 26, 5186−5192