IMS Company Profiles - Report Buyer
IMS Company Profiles - Report Buyer IMS Company Profiles - Report Buyer
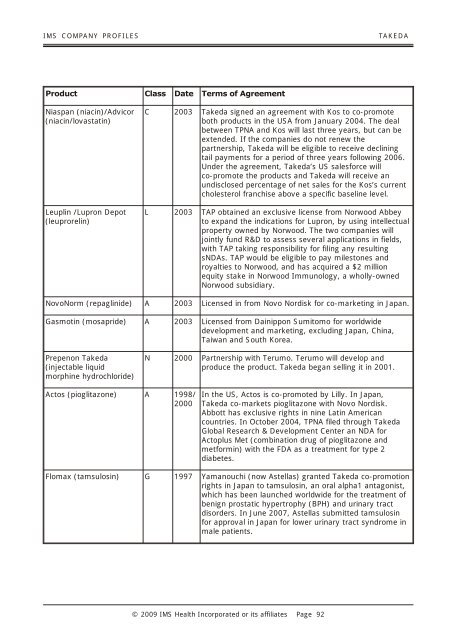
IMS COM PANY PRO FILES TAKEDA Product Class Date Terms of Agreement Niaspan (niacin)/Advicor (niacin/lovastatin) Leuplin /Lupron Depot (leuprorelin) C 2003 Takeda signed an agreement with Kos to co-promote both products in the USA from January 2004. The deal between TPNA and Kos will last three years, but can be extended. If the companies do not renew the partnership, Takeda will be eligible to receive declining tail payments for a period of three years following 2006. Under the agreement, Takeda’s US salesforce will co-promote the products and Takeda will receive an undisclosed percentage of net sales for the Kos’s current cholesterol franchise above a specific baseline level. L 2003 TAP obtained an exclusive license from Norwood Abbey to expand the indications for Lupron, by using intellectual property owned by Norwood. The two companies will jointly fund R&D to assess several applications in fields, with TAP taking responsibility for filing any resulting sNDAs. TAP would be eligible to pay milestones and royalties to Norwood, and has acquired a $2 million equity stake in Norwood Immunology, a wholly-owned Norwood subsidiary. NovoNorm (repaglinide) A 2003 Licensed in from Novo Nordisk for co-marketing in Japan. Gasmotin (mosapride) A 2003 Licensed from Dainippon Sumitomo for worldwide development and marketing, excluding Japan, China, Taiwan and South Korea. Prepenon Takeda (injectable liquid morphine hydrochloride) Actos (pioglitazone) A 1998/ 2000 N 2000 Partnership with Terumo. Terumo will develop and produce the product. Takeda began selling it in 2001. In the US, Actos is co-promoted by Lilly. In Japan, Takeda co-markets pioglitazone with Novo Nordisk. Abbott has exclusive rights in nine Latin American countries. In October 2004, TPNA filed through Takeda Global Research & Development Center an NDA for Actoplus Met (combination drug of pioglitazone and metformin) with the FDA as a treatment for type 2 diabetes. Flomax (tamsulosin) G 1997 Yamanouchi (now Astellas) granted Takeda co-promotion rights in Japan to tamsulosin, an oral alpha1 antagonist, which has been launched worldwide for the treatment of benign prostatic hypertrophy (BPH) and urinary tract disorders. In June 2007, Astellas submitted tamsulosin for approval in Japan for lower urinary tract syndrome in male patients. © 2009 IMS Health In cor po rated or its af fil i ates Page 92
IMS COM PANY PRO FILES TAKEDA Product Class Date Terms of Agreement Spectracef (cefditoren pivoxil) J 1997 TAP licensed-in exclusive rights to cefditoren pivoxil in North America from originator Meiji Seika. In 1999, Abbott obtained exclusive rights in Latin America, plus co-marketing rights in Europe (with Gruenenthal) and an option for the product in Asian countries outside Japan. However, Takeda and Abbott have now withdrawn from this deal. Benet (risedronic acid) M 1992 Originally developed by Procter & Gamble and licensed to Ajinomoto for Japan. It was then co-developed with sub-licensees Takeda and Aventis, following an agreement between P&G and Aventis to market the product worldwide. Basen (voglibose) A N/A Licensed out to Cheil for South Korea. Takepron (lansoprazole) A N/A Marketed by TAP jv with Abbott in the US, and by Takeda Pharma in German-speaking markets. Licensed out to Abbott for Canada and Latin America and to Boehringer Ingelheim and Roemmers for some Latin American countries. Almirall Prodesfarma for Spain. Wyeth for the UK, Denmark, Australia, Sweden, Norway and Pakistan. Aventis for France, Germany, the NL, Belgium, Africa and the Middle East. To Orion for Finland. To Han Il for South Korea. Blopress (candesartan cilexetil)/Blopress Comp (candesartan cilexetil/hydrochlorothiazi de) Uprima/Ixense (sublingual apomorphine) Adecut/Cupressin (delapril) C N/A Licensed out to AstraZeneca for exclusive marketing in Belgium, Denmark, Finland, the Netherlands, Norway, New Zealand, Australia, and South Africa, and co-marketing in the rest of the world outside Japan. Licensed out to Teva for Israel, Orion for Ireland, Almirall Prodesfarma for Spain, Abbott for co-marketing in Mexico, Brazil, and Argentina with AstraZeneca, and in Chile with Saval. G N/A Takeda has licensed-out the product to TAP for the USA. TAP has in turn licensed it out to Abbott for other markets. TAP holds exclusive worldwide rights to apomorphine for the treatment of Parkinson’s Disease. C N/A Licensed-out to Chiesi for Italy. Acel-Imune (DTP vaccine) J N/A Licensed out to Lederle-Praxis Biologicals (Wyeth) for markets outside Japan. © 2009 IMS Health In cor po rated or its af fil i ates Page 93
- Page 41 and 42: IMS COM PANY PRO FILES TAKEDA Li ce
- Page 43 and 44: IMS COM PANY PRO FILES TAKEDA In 20
- Page 45 and 46: IMS COM PANY PRO FILES TAKEDA three
- Page 47 and 48: IMS COM PANY PRO FILES TAKEDA (six
- Page 49 and 50: IMS COM PANY PRO FILES TAKEDA In th
- Page 51 and 52: IMS COM PANY PRO FILES TAKEDA prove
- Page 53 and 54: IMS COM PANY PRO FILES TAKEDA 1993
- Page 55 and 56: IMS COM PANY PRO FILES TAKEDA Resea
- Page 57 and 58: IMS COM PANY PRO FILES TAKEDA antia
- Page 59 and 60: IMS COM PANY PRO FILES TAKEDA down
- Page 61 and 62: IMS COM PANY PRO FILES TAKEDA where
- Page 63 and 64: IMS COM PANY PRO FILES TAKEDA TAK 4
- Page 65 and 66: IMS COM PANY PRO FILES TAKEDA spons
- Page 67 and 68: IMS COM PANY PRO FILES TAKEDA Motes
- Page 69 and 70: IMS COM PANY PRO FILES TAKEDA Li ce
- Page 71 and 72: IMS COM PANY PRO FILES TAKEDA Centr
- Page 73 and 74: IMS COM PANY PRO FILES TAKEDA sales
- Page 75 and 76: IMS COM PANY PRO FILES TAKEDA Orig
- Page 77 and 78: IMS COM PANY PRO FILES TAKEDA Sales
- Page 79 and 80: IMS COM PANY PRO FILES TAKEDA tion)
- Page 81 and 82: IMS COM PANY PRO FILES TAKEDA Sales
- Page 83 and 84: IMS COM PANY PRO FILES TAKEDA MORGA
- Page 85 and 86: IMS COM PANY PRO FILES TAKEDA IMS S
- Page 87 and 88: IMS COM PANY PRO FILES TAKEDA Pharm
- Page 89 and 90: IMS COM PANY PRO FILES TAKEDA Appen
- Page 91: IMS COM PANY PRO FILES TAKEDA Licen
- Page 95 and 96: IMS COM PANY PRO FILES TAKEDA R&D P
- Page 97 and 98: IMS COM PANY PRO FILES TAKEDA Produ
- Page 99 and 100: IMS COM PANY PRO FILES TAKEDA Gener
- Page 101 and 102: IMS COM PANY PRO FILES TAKEDA Compa
- Page 103 and 104: IMS COM PANY PRO FILES TAKEDA Compa
- Page 105 and 106: IMS COM PANY PRO FILES TAKEDA Compa
- Page 107 and 108: IMS COM PANY PRO FILES TAKEDA Compa
- Page 109 and 110: IMS COM PANY PRO FILES TAKEDA March
- Page 111 and 112: IMS COM PANY PRO FILES TAKEDA • Y
- Page 113 and 114: IMS COM PANY PRO FILES TAKEDA • Y
- Page 115 and 116: IMS COM PANY PRO FILES TAKEDA 420 e
- Page 117 and 118: IMS COM PANY PRO FILES TAKEDA • Y
- Page 119 and 120: IMS COM PANY PRO FILES TAKEDA Taked
- Page 121 and 122: IMS COM PANY PRO FILES TAKEDA • Y
- Page 123 and 124: IMS COM PANY PRO FILES TAKEDA SUBSI
- Page 125 and 126: IMS COM PANY PRO FILES TAKEDA SUBSI
- Page 127 and 128: IMS COM PANY PRO FILES TAKEDA SUBSI
- Page 129 and 130: IMS COM PANY PRO FILES TAKEDA SUBSI
- Page 131 and 132: IMS COM PANY PRO FILES TAKEDA SUBSI
<strong>IMS</strong> COM PANY PRO FILES TAKEDA<br />
Product Class Date Terms of Agreement<br />
Niaspan (niacin)/Advicor<br />
(niacin/lovastatin)<br />
Leuplin /Lupron Depot<br />
(leuprorelin)<br />
C 2003 Takeda signed an agreement with Kos to co-promote<br />
both products in the USA from January 2004. The deal<br />
between TPNA and Kos will last three years, but can be<br />
extended. If the companies do not renew the<br />
partnership, Takeda will be eligible to receive declining<br />
tail payments for a period of three years following 2006.<br />
Under the agreement, Takeda’s US salesforce will<br />
co-promote the products and Takeda will receive an<br />
undisclosed percentage of net sales for the Kos’s current<br />
cholesterol franchise above a specific baseline level.<br />
L 2003 TAP obtained an exclusive license from Norwood Abbey<br />
to expand the indications for Lupron, by using intellectual<br />
property owned by Norwood. The two companies will<br />
jointly fund R&D to assess several applications in fields,<br />
with TAP taking responsibility for filing any resulting<br />
sNDAs. TAP would be eligible to pay milestones and<br />
royalties to Norwood, and has acquired a $2 million<br />
equity stake in Norwood Immunology, a wholly-owned<br />
Norwood subsidiary.<br />
NovoNorm (repaglinide) A 2003 Licensed in from Novo Nordisk for co-marketing in Japan.<br />
Gasmotin (mosapride) A 2003 Licensed from Dainippon Sumitomo for worldwide<br />
development and marketing, excluding Japan, China,<br />
Taiwan and South Korea.<br />
Prepenon Takeda<br />
(injectable liquid<br />
morphine hydrochloride)<br />
Actos (pioglitazone) A 1998/<br />
2000<br />
N 2000 Partnership with Terumo. Terumo will develop and<br />
produce the product. Takeda began selling it in 2001.<br />
In the US, Actos is co-promoted by Lilly. In Japan,<br />
Takeda co-markets pioglitazone with Novo Nordisk.<br />
Abbott has exclusive rights in nine Latin American<br />
countries. In October 2004, TPNA filed through Takeda<br />
Global Research & Development Center an NDA for<br />
Actoplus Met (combination drug of pioglitazone and<br />
metformin) with the FDA as a treatment for type 2<br />
diabetes.<br />
Flomax (tamsulosin) G 1997 Yamanouchi (now Astellas) granted Takeda co-promotion<br />
rights in Japan to tamsulosin, an oral alpha1 antagonist,<br />
which has been launched worldwide for the treatment of<br />
benign prostatic hypertrophy (BPH) and urinary tract<br />
disorders. In June 2007, Astellas submitted tamsulosin<br />
for approval in Japan for lower urinary tract syndrome in<br />
male patients.<br />
© 2009 <strong>IMS</strong> Health In cor po rated or its af fil i ates Page 92