Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
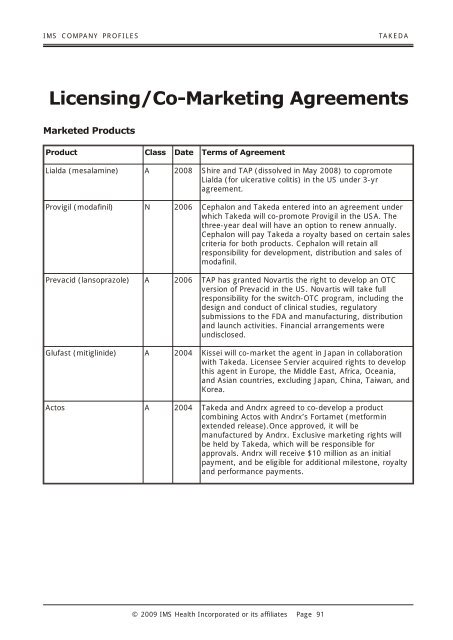
<strong>IMS</strong> COM PANY PRO FILES TAKEDA<br />
Licensing/Co-Marketing Agreements<br />
Marketed Products<br />
Product Class Date Terms of Agreement<br />
Lialda (mesalamine) A 2008 Shire and TAP (dissolved in May 2008) to copromote<br />
Lialda (for ulcerative colitis) in the US under 3-yr<br />
agreement.<br />
Provigil (modafinil) N 2006 Cephalon and Takeda entered into an agreement under<br />
which Takeda will co-promote Provigil in the USA. The<br />
three-year deal will have an option to renew annually.<br />
Cephalon will pay Takeda a royalty based on certain sales<br />
criteria for both products. Cephalon will retain all<br />
responsibility for development, distribution and sales of<br />
modafinil.<br />
Prevacid (lansoprazole) A 2006 TAP has granted Novartis the right to develop an OTC<br />
version of Prevacid in the US. Novartis will take full<br />
responsibility for the switch-OTC program, including the<br />
design and conduct of clinical studies, regulatory<br />
submissions to the FDA and manufacturing, distribution<br />
and launch activities. Financial arrangements were<br />
undisclosed.<br />
Glufast (mitiglinide) A 2004 Kissei will co-market the agent in Japan in collaboration<br />
with Takeda. Licensee Servier acquired rights to develop<br />
this agent in Europe, the Middle East, Africa, Oceania,<br />
and Asian countries, excluding Japan, China, Taiwan, and<br />
Korea.<br />
Actos A 2004 Takeda and Andrx agreed to co-develop a product<br />
combining Actos with Andrx’s Fortamet (metformin<br />
extended release).Once approved, it will be<br />
manufactured by Andrx. Exclusive marketing rights will<br />
be held by Takeda, which will be responsible for<br />
approvals. Andrx will receive $10 million as an initial<br />
payment, and be eligible for additional milestone, royalty<br />
and performance payments.<br />
© 2009 <strong>IMS</strong> Health In cor po rated or its af fil i ates Page 91