European Journal of Scientific Research - EuroJournals
European Journal of Scientific Research - EuroJournals
European Journal of Scientific Research - EuroJournals
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
335 S. J. Fatemi, A. Badiei and A. Hooshmand<br />
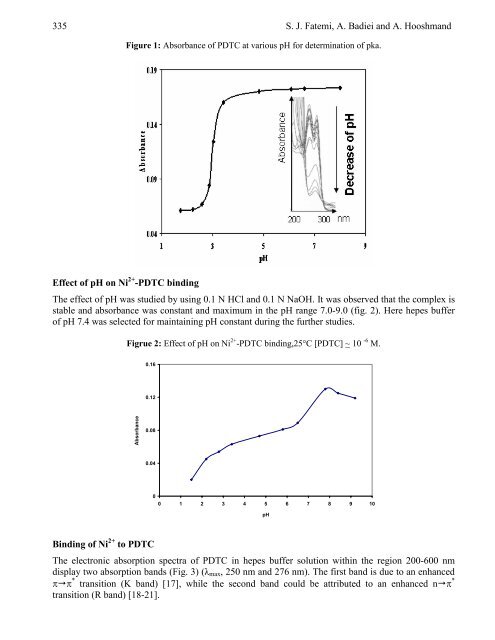
Figure 1: Absorbance <strong>of</strong> PDTC at various pH for determination <strong>of</strong> pka.<br />
Effect <strong>of</strong> pH on Ni 2+ -PDTC binding<br />
The effect <strong>of</strong> pH was studied by using 0.1 N HCl and 0.1 N NaOH. It was observed that the complex is<br />
stable and absorbance was constant and maximum in the pH range 7.0-9.0 (fig. 2). Here hepes buffer<br />
<strong>of</strong> pH 7.4 was selected for maintaining pH constant during the further studies.<br />
Figrue 2: Effect <strong>of</strong> pH on Ni 2+ -PDTC binding,25°C [PDTC] ~ 10 -6 M.<br />
Absorbance<br />
0.16<br />
0.12<br />
0.08<br />
0.04<br />
Binding <strong>of</strong> Ni 2+ to PDTC<br />
0<br />
0 1 2 3 4 5<br />
pH<br />
6 7 8 9 10<br />
The electronic absorption spectra <strong>of</strong> PDTC in hepes buffer solution within the region 200-600 nm<br />
display two absorption bands (Fig. 3) (λmax, 250 nm and 276 nm). The first band is due to an enhanced<br />
π�π * transition (K band) [17], while the second band could be attributed to an enhanced n�π *<br />
transition (R band) [18-21].