Regioselectivity of the Reactions of Heteroatom-Stabilized Allyl ...
Regioselectivity of the Reactions of Heteroatom-Stabilized Allyl ...
Regioselectivity of the Reactions of Heteroatom-Stabilized Allyl ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
700<br />
Scheme 123<br />
Scheme 124<br />
E. Nitrogen and Phosphorus<br />
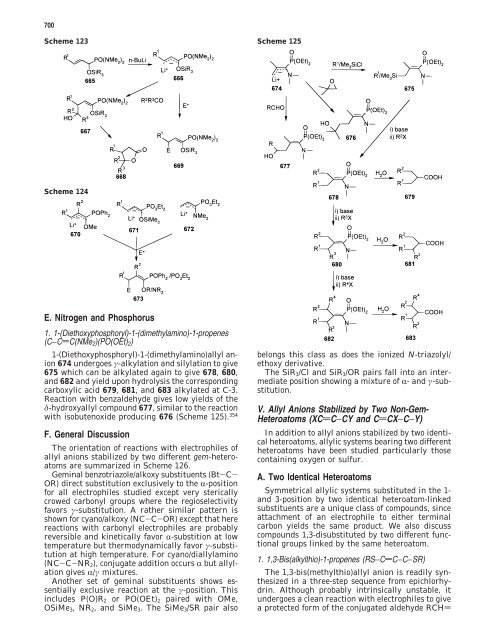
1. 1-(Diethoxyphosphoryl)-1-(dimethylamino)-1-propenes<br />
(C−CdC(NMe2)(PO(OEt)2)<br />
1-(Diethoxyphosphoryl)-1-(dimethylamino)allyl anion<br />
674 undergoes γ-alkylation and silylation to give<br />
675 which can be alkylated again to give 678, 680,<br />
and 682 and yield upon hydrolysis <strong>the</strong> corresponding<br />
carboxylic acid 679, 681, and 683 alkylated at C-3.<br />
Reaction with benzaldehyde gives low yields <strong>of</strong> <strong>the</strong><br />
δ-hydroxyallyl compound 677, similar to <strong>the</strong> reaction<br />
with isobutenoxide producing 676 (Scheme 125). 354<br />
F. General Discussion<br />
The orientation <strong>of</strong> reactions with electrophiles <strong>of</strong><br />
allyl anions stabilized by two different gem-heteroatoms<br />
are summarized in Scheme 126.<br />
Geminal benzotriazole/alkoxy substituents (Bt-C-<br />
OR) direct substitution exclusively to <strong>the</strong> R-position<br />
for all electrophiles studied except very sterically<br />
crowed carbonyl groups where <strong>the</strong> regioselectivity<br />
favors γ-substitution. A ra<strong>the</strong>r similar pattern is<br />
shown for cyano/alkoxy (NC-C-OR) except that here<br />
reactions with carbonyl electrophiles are probably<br />
reversible and kinetically favor R-substition at low<br />
temperature but <strong>the</strong>rmodynamically favor γ-substitution<br />
at high temperature. For cyano/diallylamino<br />
(NC-C-NR2), conjugate addition occurs R but allylation<br />
gives R/γ mixtures.<br />
Ano<strong>the</strong>r set <strong>of</strong> geminal substituents shows essentially<br />
exclusive reaction at <strong>the</strong> γ-position. This<br />
includes P(O)R2 or PO(OEt)2 paired with OMe,<br />
OSiMe3, NR2, and SiMe3. The SiMe3/SR pair also<br />
Scheme 125<br />
belongs this class as does <strong>the</strong> ionized N-triazolyl/<br />
ethoxy derivative.<br />
The SiR3/Cl and SiR3/OR pairs fall into an intermediate<br />
position showing a mixture <strong>of</strong> R- and γ-substitution.<br />
V. <strong>Allyl</strong> Anions <strong>Stabilized</strong> by Two Non-Gem-<br />
<strong>Heteroatom</strong>s (XCdC−CY and CdCX−C−Y)<br />
In addition to allyl anions stabilized by two identical<br />
heteroatoms, allylic systems bearing two different<br />
heteroatoms have been studied particularly those<br />
containing oxygen or sulfur.<br />
A. Two Identical <strong>Heteroatom</strong>s<br />
Symmetrical allylic systems substituted in <strong>the</strong> 1and<br />
3-position by two identical heteroatom-linked<br />
substituents are a unique class <strong>of</strong> compounds, since<br />
attachment <strong>of</strong> an electrophile to ei<strong>the</strong>r terminal<br />
carbon yields <strong>the</strong> same product. We also discuss<br />
compounds 1,3-disubstituted by two different functional<br />
groups linked by <strong>the</strong> same heteroatom.<br />
1. 1,3-Bis(alkylthio)-1-propenes (RS−CdC−C−SR)<br />
The 1,3-bis(methylthio)allyl anion is readily syn<strong>the</strong>sized<br />
in a three-step sequence from epichlorhydrin.<br />
Although probably intrinsically unstable, it<br />
undergoes a clean reaction with electrophiles to give<br />
a protected form <strong>of</strong> <strong>the</strong> conjugated aldehyde RCHd


![and 1-Amido-3-alkylimidazo[1,5-a]pyridines - Ark.chem.ufl.edu ...](https://img.yumpu.com/19437237/1/190x245/and-1-amido-3-alkylimidazo15-apyridines-arkchemufledu-.jpg?quality=85)











