National Cancer Institute - NCI Division of Cancer Treatment and ...
National Cancer Institute - NCI Division of Cancer Treatment and ... National Cancer Institute - NCI Division of Cancer Treatment and ...
DEVELOPMENTAL THERAPEUTICS PROGRAM The Developmental Therapeutics Program has been involved in the discovery or development of more than 70 percent of the anticancer therapeutics on the market today. The Developmental Therapeutics Program (DTP) has played an intimate role in the discovery or development of 40 U.S.-licensed chemotherapeutic agents, with the rest coming directly from the pharmaceutical industry. DTP’s roster of drug success stories is impressive. On that list is paclitaxel (Taxol®), one of the most widely prescribed anticancer drugs on the market. Paclitaxel, a natural product, was first harvested by researchers working under a joint U.S. Department of Agriculture- National Cancer Institute (NCI) grant. It was a DTP contractor who formulated the drug for use in clinical trials. Bortezomib (Velcade®) is another DTP success story. In cooperation with its commercial sponsor, bortezomib was screened and formulated by DTP. Approved by the FDA in 2003, it was the first treatment in more than a decade to be approved for patients with multiple myeloma. DTP has been involved in the discovery or development of more than 70 percent of the anticancer therapeutics on the market today. Although many academic and private-industry laboratories also are focused on drug discovery, financial and technical burdens as well as lack of funding and infrastructure present barriers that may keep promising therapeutics from O V E R V I E W reaching patients. DTP helps to overcome therapeutic development barriers by supporting high-risk projects. In keeping with its goal to turn molecules into medicine for the public health, DTP, created by Congress in 1955 as the Cancer Chemotherapy National Service Center, serves as a vital resource in acquiring preclinical information; providing research materials, including Web-accessible data and tools, vialed and plated compounds, tumor cells, and animals; and providing bulk drugs for investigational new drug (IND)–directed studies. Dr. Jerry M. Collins, Associate Director Jerry M. Collins, Ph.D., is an internationally recognized pharmacologist. He has been closely associated with NCI’s drug development efforts for more than 25 years, first as an NCI intramural investigator and then as the Chief of the Pharmacokinetics Section. From 1988 until 2005, Dr. Collins served as the Director of the FDA’s Laboratory of Clinical Pharmacology, where he headed the development of new methods to facilitate research on human tissue metabolism to create an in vitro model to reduce adverse drug reactions. Dr. Collins was named Associate Director of the DCTD Developmental Therapeutics Program in September 2005. Dr. Collins’ areas of expertise are clinical pharmacology, the application of pharmacokinetic and pharmacodynamic (PK/PD) principles to cancer research, and increasing biomarker efficacy with positron emission tomography. Dr. Collins received his bachelor’s degree from Drexel University and his master’s and doctoral degrees from the University of Pennsylvania. He is the author or co-author of more than 170 articles and holds eight patents. D E V E L O P M E N T A L T H E R A P E U T I C S P R O G R A M ■ 95
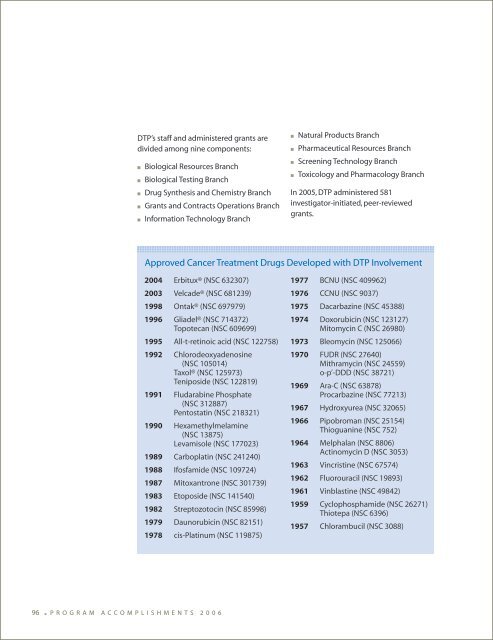
DTP’s staff and administered grants are divided among nine components: ■ Biological Resources Branch ■ Biological Testing Branch ■ Drug Synthesis and Chemistry Branch ■ Grants and Contracts Operations Branch ■ Information Technology Branch 2004 Erbitux® (NSC 632307) 2003 Velcade® (NSC 681239) 1998 Ontak® (NSC 697979) 1996 Gliadel® (NSC 714372) Topotecan (NSC 609699) 1995 All-t-retinoic acid (NSC 122758) 1992 Chlorodeoxyadenosine (NSC 105014) Taxol® (NSC 125973) Teniposide (NSC 122819) 1991 Fludarabine Phosphate (NSC 312887) Pentostatin (NSC 218321) 1990 Hexamethylmelamine (NSC 13875) Levamisole (NSC 177023) 1989 Carboplatin (NSC 241240) 1988 Ifosfamide (NSC 109724) 1987 Mitoxantrone (NSC 301739) 1983 Etoposide (NSC 141540) 1982 Streptozotocin (NSC 85998) 1979 Daunorubicin (NSC 82151) 1978 cis-Platinum (NSC 119875) 96 ■ P R O G R A M A C C O M P L I S H M E N T S 2 0 0 6 ■ Natural Products Branch ■ Pharmaceutical Resources Branch ■ Screening Technology Branch ■ Toxicology and Pharmacology Branch In 2005, DTP administered 581 investigator-initiated, peer-reviewed grants. Approved Cancer Treatment Drugs Developed with DTP Involvement 1977 BCNU (NSC 409962) 1976 CCNU (NSC 9037) 1975 Dacarbazine (NSC 45388) 1974 Doxorubicin (NSC 123127) Mitomycin C (NSC 26980) 1973 Bleomycin (NSC 125066) 1970 FUDR (NSC 27640) Mithramycin (NSC 24559) o-p’-DDD (NSC 38721) 1969 Ara-C (NSC 63878) Procarbazine (NSC 77213) 1967 Hydroxyurea (NSC 32065) 1966 Pipobroman (NSC 25154) Thioguanine (NSC 752) 1964 Melphalan (NSC 8806) Actinomycin D (NSC 3053) 1963 Vincristine (NSC 67574) 1962 Fluorouracil (NSC 19893) 1961 Vinblastine (NSC 49842) 1959 Cyclophosphamide (NSC 26271) Thiotepa (NSC 6396) 1957 Chlorambucil (NSC 3088)
- Page 49 and 50: Progress in many areas of cancer re
- Page 51 and 52: Diagnostic Imaging Program in 1996,
- Page 53 and 54: NCI Visuals Online, Terese Winslow,
- Page 55 and 56: ■ Support development and deliver
- Page 57 and 58: Small Animal Imaging Resource Progr
- Page 59 and 60: C U R R E N T F U N D I N G O P P O
- Page 61 and 62: Quick-Trials for Imaging and Image-
- Page 63 and 64: Association of American Cancer Inst
- Page 65 and 66: Food and Drug Administration and Ce
- Page 67 and 68: Dr. Edward A. Neuwelt, Oregon Healt
- Page 69 and 70: Virtual Colonoscopy Training Collec
- Page 71 and 72: Not only does CTEP identify promisi
- Page 73 and 74: The Clinical Trials Cooperative Gro
- Page 75 and 76: of pediatric cancers and therapeuti
- Page 77 and 78: the front line treatment of chronic
- Page 79 and 80: Quick-Trials for Novel Cancer Thera
- Page 81 and 82: Industry Collaborators Agent Name 7
- Page 83 and 84: Industry Collaborators Agent Name 7
- Page 85 and 86: NCI Visuals Online, George McGregor
- Page 87 and 88: provided for use in this clinical t
- Page 89 and 90: with or without bevacizumab (NSC #
- Page 91 and 92: Oxaliplatin-Based Regimen Permits S
- Page 93 and 94: egular checkups to look for lymph-n
- Page 95 and 96: NCI Visuals Online. Human lymphoma
- Page 97 and 98: T O O L S , P R O D U C T S , A N D
- Page 99: ■ Clinical Trials Monitoring Bran
- Page 103 and 104: therapeutics. Proposed exploratory
- Page 105 and 106: M A J O R O N G O I N G I N I T I A
- Page 107 and 108: National Cancer Institute. discover
- Page 109 and 110: C U R R E N T F U N D I N G O P P O
- Page 111 and 112: DTP oversees animal-production faci
- Page 113 and 114: disease can require considerable in
- Page 115 and 116: National Cancer Institute. commerci
- Page 117 and 118: The Type 1 Diabetes Rapid Access to
- Page 119 and 120: H I S T O R Y - M A R K I N G E V E
- Page 121 and 122: With this approach, patients are no
- Page 123 and 124: This included a camptothecin deriva
- Page 125 and 126: RRP supports research involving a v
- Page 127 and 128: Laredo Medical Center, Laredo, TX h
- Page 129 and 130: With TELESYNERGY® as a link, healt
- Page 131 and 132: Radiation Bioterrorism Research and
- Page 133 and 134: Enhanced Radiosensitivity The Molec
- Page 135 and 136: M E E T I N G S A N D W O R K S H O
- Page 137 and 138: Cancer Imaging Program 132 ■ P R
- Page 139 and 140: Cancer Therapy Evaluation Program,
- Page 141 and 142: Developmental Therapeutics Program,
- Page 143 and 144: Developmental Therapeutics Program,
DTP’s staff <strong>and</strong> administered grants are<br />
divided among nine components:<br />
■ Biological Resources Branch<br />
■ Biological Testing Branch<br />
■ Drug Synthesis <strong>and</strong> Chemistry Branch<br />
■ Grants <strong>and</strong> Contracts Operations Branch<br />
■ Information Technology Branch<br />
2004 Erbitux® (NSC 632307)<br />
2003 Velcade® (NSC 681239)<br />
1998 Ontak® (NSC 697979)<br />
1996 Gliadel® (NSC 714372)<br />
Topotecan (NSC 609699)<br />
1995 All-t-retinoic acid (NSC 122758)<br />
1992 Chlorodeoxyadenosine<br />
(NSC 105014)<br />
Taxol® (NSC 125973)<br />
Teniposide (NSC 122819)<br />
1991 Fludarabine Phosphate<br />
(NSC 312887)<br />
Pentostatin (NSC 218321)<br />
1990 Hexamethylmelamine<br />
(NSC 13875)<br />
Levamisole (NSC 177023)<br />
1989 Carboplatin (NSC 241240)<br />
1988 Ifosfamide (NSC 109724)<br />
1987 Mitoxantrone (NSC 301739)<br />
1983 Etoposide (NSC 141540)<br />
1982 Streptozotocin (NSC 85998)<br />
1979 Daunorubicin (NSC 82151)<br />
1978 cis-Platinum (NSC 119875)<br />
96 ■ P R O G R A M A C C O M P L I S H M E N T S 2 0 0 6<br />
■ Natural Products Branch<br />
■ Pharmaceutical Resources Branch<br />
■ Screening Technology Branch<br />
■ Toxicology <strong>and</strong> Pharmacology Branch<br />
In 2005, DTP administered 581<br />
investigator-initiated, peer-reviewed<br />
grants.<br />
Approved <strong>Cancer</strong> <strong>Treatment</strong> Drugs Developed with DTP Involvement<br />
1977 BCNU (NSC 409962)<br />
1976 CCNU (NSC 9037)<br />
1975 Dacarbazine (NSC 45388)<br />
1974 Doxorubicin (NSC 123127)<br />
Mitomycin C (NSC 26980)<br />
1973 Bleomycin (NSC 125066)<br />
1970 FUDR (NSC 27640)<br />
Mithramycin (NSC 24559)<br />
o-p’-DDD (NSC 38721)<br />
1969 Ara-C (NSC 63878)<br />
Procarbazine (NSC 77213)<br />
1967 Hydroxyurea (NSC 32065)<br />
1966 Pipobroman (NSC 25154)<br />
Thioguanine (NSC 752)<br />
1964 Melphalan (NSC 8806)<br />
Actinomycin D (NSC 3053)<br />
1963 Vincristine (NSC 67574)<br />
1962 Fluorouracil (NSC 19893)<br />
1961 Vinblastine (NSC 49842)<br />
1959 Cyclophosphamide (NSC 26271)<br />
Thiotepa (NSC 6396)<br />
1957 Chlorambucil (NSC 3088)