chapter 2 palladium catalysts in suzuki cross- coupling reaction
chapter 2 palladium catalysts in suzuki cross- coupling reaction
chapter 2 palladium catalysts in suzuki cross- coupling reaction
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Smith et al <strong>in</strong>troduced the use of Pd(II)-exchanged perovskites (LaFe0.57Co0.38<br />
Pd0.05O3) as a new class of <strong>catalysts</strong> <strong>in</strong> the Suzuki coupl<strong>in</strong>g of 2-bromoanisole, 2-bromo<br />
pyrid<strong>in</strong>e and arylboronic acids with low levels of Pd leach<strong>in</strong>g at 80 °C (Smith et al.<br />
2003).<br />
Zeolites, which have specific properties because of their well-def<strong>in</strong>ed structure<br />
and pores with molecular dimensions, have been also used as a support material (Smith<br />
and Nothesisz 1995).<br />
3.3. Zeolite supported <strong>palladium</strong> <strong>catalysts</strong> <strong>in</strong> the Suzuki Reactions<br />
3.3.1. Structural Features of Zeolite<br />
Zeolites are three-dimensional, microporous, crystall<strong>in</strong>e solids with well-def<strong>in</strong>ed<br />
structures that conta<strong>in</strong> alum<strong>in</strong>um, silicon, and oxygen <strong>in</strong> their regular framework;<br />
cations and water are located <strong>in</strong> the pores. Zeolites have a uniform pore structure<br />
(Weitkamp and Puppe 1999).<br />
The formula of zeolite is written as M2/n. Al2O3. xSiO2. yH2O where n is the<br />
cation valence; M is the cation which balances the negative charge.<br />
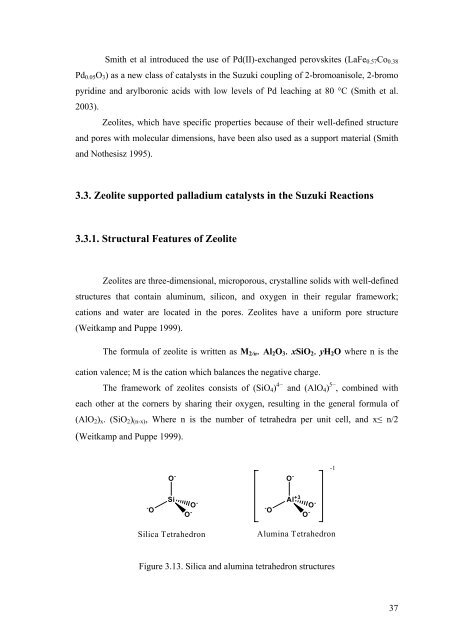
The framework of zeolites consists of (SiO4) 4− and (AlO4) 5− , comb<strong>in</strong>ed with<br />
each other at the corners by shar<strong>in</strong>g their oxygen, result<strong>in</strong>g <strong>in</strong> the general formula of<br />
(AlO2)x. (SiO2)(n-x), Where n is the number of tetrahedra per unit cell, and x≤ n/2<br />
(Weitkamp and Puppe 1999).<br />
- O<br />
O -<br />
Si<br />
O- O- - O<br />
O -<br />
Al +3<br />
O- O- Silica Tetrahedron Alum<strong>in</strong>a Tetrahedron<br />
Figure 3.13. Silica and alum<strong>in</strong>a tetrahedron structures<br />
-1<br />
37