3. FOOD ChEMISTRy & bIOTEChNOLOGy 3.1. Lectures
3. FOOD ChEMISTRy & bIOTEChNOLOGy 3.1. Lectures
3. FOOD ChEMISTRy & bIOTEChNOLOGy 3.1. Lectures
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chem. Listy, 102, s265–s1311 (2008) Food Chemistry & Biotechnology<br />
V a c u u m D i s t i l l a t i o n o f T O M E<br />
Data on amounts of obtained distillates (D1, D2), residues<br />
(Z1, Z2) and weight losses (WL) from 1 st and 2 nd distillation<br />
stage are listed in Table V and VI.<br />
Table V<br />
Vacuum Distillation material balance: 1 st stage<br />
TOME D1 Z1 WL<br />
[g] [g] [wt. %] [g] [wt. %] [wt. %]<br />
2362 845 35.8 1419 60.0 4.2<br />
Table VI<br />
Vacuum distillation material balance: 2 nd stage<br />
TOME D2 Z2 WL<br />
[g] [g] [wt. %] [g] [wt. %] [wt. %]<br />
845.3 712 84.2 101.4 12.0 <strong>3.</strong>8<br />
The prepared amount of TOME was heated and then let<br />
settle. Formed water and unreacted methanol were removed<br />
with a vacuum (molecular) evaporator.<br />
Then 2.5 dm 3 TOME was withdrawn from 3 dm 3 volume<br />
of TOME and distillated at the folowing conditions: pressure<br />
10–50 Pa and temperature 135–140 °C. 955 ml (~ 845 g) of<br />
distillate (D1) was obtained which represented ~ 38 % vol.<br />
(~ 36 % wt.). During this distillation D1 was polluted<br />
by RA.<br />
Destillate D1 955 ml (~ 845 g) was consequently distillated<br />
at pressure 2–20 Pa and temperature 130–135 °C.<br />
Under these conditions, we obtained 835 ml (711.8 g) of distillate<br />
D2, i.e. ~ 87 % vol. from D1, (84 % wt.)<br />
The total yield of these processes: from 2,500 ml TOME<br />
(2,363 g) we obtained 835 ml (711.8 g) which represented<br />
3<strong>3.</strong>4 % vol. (30.1 % wt.).<br />
Acid number parameter of D2 (19.610 mg KOH g –1 ) did<br />
not comply with European standard En 14214 for biodiesel<br />
(stipulating less than 0.5 mg KOH g –1 ), D2 had to undergo<br />
further treatment. D2 included some RA. They were neutralised<br />
and precipitated and D2 (tall oil fatty acids methyl esthers<br />
– TOFAME) after this treatment had acid number 0.3 mg<br />
KOH g –1 . This value fully meets En 14214. However, total<br />
volume of the final product decreased from 835 to 630 ml<br />
(566.6 g) which represented – when comparing to the TOME<br />
amount – 25.2 % vol., (~ 24 % wt.).<br />
The final product prepared in such a mode was subjected<br />
to analyses in laboratory of VÚRUP, which was accredited<br />
for analyses of biofuels. Results are assembled in Table VII.<br />
R e s u l t s O b t a i n e d b y F o u r i e r<br />
T r a n s f o r m I n f r a r e d S p e c t r o m e t r y<br />
( F T I R )<br />
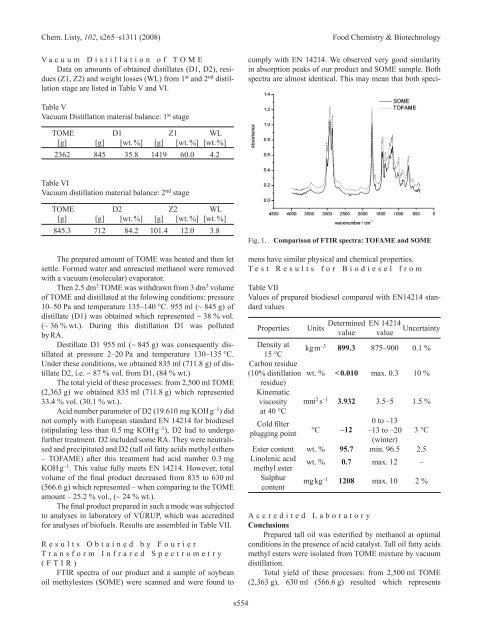
FTIR spectra of our product and a sample of soybean<br />
oil methylesters (SOME) were scanned and were found to<br />
s554<br />
comply with En 14214. We observed very good similarity<br />
in absorption peaks of our product and SOME sample. Both<br />
spectra are almost identical. This may mean that both speci-<br />
Fig. 1. Comparison of FTIR spectra: TOFAME and SOME<br />
mens have similar physical and chemical properties.<br />
T e s t R e s u l t s f o r B i o d i e s e l f r o m<br />
Table VII<br />
Values of prepared biodiesel compared with En14214 standard<br />
values<br />
Properties Units Determined En 14214 Uncertainty<br />
value value<br />
Density at<br />
kg m –3 15 °C<br />
Carbon residue<br />
899.3 875–900 0.1 %<br />
(10% distillation wt. %<br />
residue)<br />
Kinematic<br />
< 0.010 max. 0.3 10 %<br />
mm2 s –1 viscosity<br />
at 40 °C<br />
<strong>3.</strong>932 <strong>3.</strong>5–5 1.5 %<br />
Cold filter<br />
plugging point<br />
°C –12<br />
0 to –13<br />
–13 to –20<br />
(winter)<br />
3 °C<br />
Ester content wt. % 95.7 min. 96.5 2.5<br />
Linolenic acid<br />
methyl ester<br />
wt. % 0.7 max. 12 –<br />
Sulphur<br />
mg kg –1 content<br />
1208 max. 10 2 %<br />
A c c r e d i t e d L a b o r a t o r y<br />
Conclusions<br />
Prepared tall oil was esterified by methanol at optimal<br />
conditions in the presence of acid catalyst. Tall oil fatty acids<br />
methyl esters were isolated from TOME mixture by vacuum<br />
distillation.<br />
Total yield of these processes: from 2,500 ml TOME<br />
(2,363 g), 630 ml (566.6 g) resulted which represents