"Front Matter". In: Organosilanes in Radical Chemistry - Index of
"Front Matter". In: Organosilanes in Radical Chemistry - Index of
"Front Matter". In: Organosilanes in Radical Chemistry - Index of
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Carbon–Carbon Double Bonds 93<br />
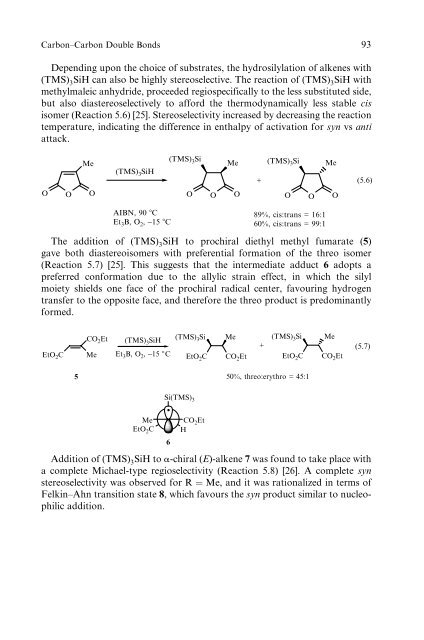
Depend<strong>in</strong>g upon the choice <strong>of</strong> substrates, the hydrosilylation <strong>of</strong> alkenes with<br />
(TMS) 3SiH can also be highly stereoselective. The reaction <strong>of</strong> (TMS) 3SiH with<br />
methylmaleic anhydride, proceeded regiospecifically to the less substituted side,<br />
but also diastereoselectively to afford the thermodynamically less stable cis<br />
isomer (Reaction 5.6) [25]. Stereoselectivity <strong>in</strong>creased by decreas<strong>in</strong>g the reaction<br />
temperature, <strong>in</strong>dicat<strong>in</strong>g the difference <strong>in</strong> enthalpy <strong>of</strong> activation for syn vs anti<br />
attack.<br />
O<br />
O<br />
Me<br />
O<br />
(TMS) 3SiH<br />
AIBN, 90 �C<br />
Et 3B, O 2, −15 �C<br />
(TMS) 3 Si<br />
O<br />
O<br />
Me<br />
O<br />
(TMS) 3Si<br />
O<br />
O<br />
89%, cis:trans = 16:1<br />
60%, cis:trans = 99:1<br />
Me<br />
+ (5.6)<br />
The addition <strong>of</strong> (TMS) 3SiH to prochiral diethyl methyl fumarate (5)<br />
gave both diastereoisomers with preferential formation <strong>of</strong> the threo isomer<br />
(Reaction 5.7) [25]. This suggests that the <strong>in</strong>termediate adduct 6 adopts a<br />
preferred conformation due to the allylic stra<strong>in</strong> effect, <strong>in</strong> which the silyl<br />
moiety shields one face <strong>of</strong> the prochiral radical center, favour<strong>in</strong>g hydrogen<br />
transfer to the opposite face, and therefore the threo product is predom<strong>in</strong>antly<br />
formed.<br />
EtO 2 C<br />
CO 2 Et (TMS) 3SiH<br />
Me<br />
Et 3B, O 2, −15 �C<br />
Me<br />
EtO2C (TMS) 3 Si<br />
Si(TMS) 3<br />
EtO 2C<br />
CO2Et H<br />
Me<br />
CO 2Et<br />
+<br />
(TMS) 3 Si<br />
EtO 2 C<br />
5 50%, threo:erythro = 45:1<br />
6<br />
Me<br />
O<br />
CO 2Et<br />
Addition <strong>of</strong> (TMS) 3SiH to a-chiral (E)-alkene 7 was found to take place with<br />
a complete Michael-type regioselectivity (Reaction 5.8) [26]. A complete syn<br />
stereoselectivity was observed for R ¼ Me, and it was rationalized <strong>in</strong> terms <strong>of</strong><br />
Felk<strong>in</strong>–Ahn transition state 8, which favours the syn product similar to nucleophilic<br />
addition.<br />
(5.7)