"Front Matter". In: Organosilanes in Radical Chemistry - Index of
"Front Matter". In: Organosilanes in Radical Chemistry - Index of "Front Matter". In: Organosilanes in Radical Chemistry - Index of
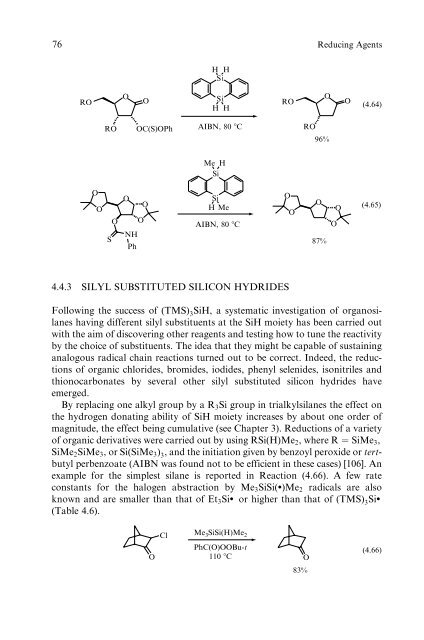
76 Reducing Agents RO O O O O RO OC(S)OPh S O O NH Ph O O H H Si Si H H AIBN, 80 �C Me H Si Si HMe AIBN, 80 �C 4.4.3 SILYL SUBSTITUTED SILICON HYDRIDES RO O O RO O 96% O 87% O O O (4.64) (4.65) Following the success of (TMS) 3SiH, a systematic investigation of organosilanes having different silyl substituents at the SiH moiety has been carried out with the aim of discovering other reagents and testing how to tune the reactivity by the choice of substituents. The idea that they might be capable of sustaining analogous radical chain reactions turned out to be correct. Indeed, the reductions of organic chlorides, bromides, iodides, phenyl selenides, isonitriles and thionocarbonates by several other silyl substituted silicon hydrides have emerged. By replacing one alkyl group by a R3Si group in trialkylsilanes the effect on the hydrogen donating ability of SiH moiety increases by about one order of magnitude, the effect being cumulative (see Chapter 3). Reductions of a variety of organic derivatives were carried out by using RSi(H)Me2, where R ¼ SiMe3, SiMe2SiMe3, or Si(SiMe3) 3, and the initiation given by benzoyl peroxide or tertbutyl perbenzoate (AIBN was found not to be efficient in these cases) [106]. An example for the simplest silane is reported in Reaction (4.66). A few rate constants for the halogen abstraction by Me3SiSi(:)Me2 radicals are also known and are smaller than that of Et3Si: or higher than that of (TMS) 3Si: (Table 4.6). Cl Me3SiSi(H)Me2 PhC(O)OOBu-t O 110 �C O 83% (4.66)
Other Silicon Hydrides 77 Table 4.6 Rate constants at 27 8C for the reaction of Me3SiSi(:)Me2 radicals with a few halides [107] Halide k=M 1 s 1 (CH3) 3CCl 4:2 10 5 CH3(CH2) 4Br 1:6 10 8 (CH3) 3CBr 2:6 10 8 Reduction of a variety of organic derivatives was carried out by using (Me3Si) 2Si(H)Me under normal conditions, i.e., AIBN at 80 8C [108]. The mono-reduction of a gem-dichloride is shown as an example in Reaction (4.67). With two silyl substituents, (Me3Si) 2Si(H)Me is an effective reducing agent which allows the formation of the desired product to be favoured due to a slower hydrogen transfer. Cl Me Cl (Me3Si) 2Si(H)Me CO2Me AIBN, 80 �C Cl H Me CO 2 Me 93% (4.67) Poly(phenylsilane)s of the type H(RSiH) nH, where R ¼ n-hexyl or phenyl, have been used as radical-based reducing agents for organic halides [109]. They rival the effectiveness of (TMS) 3SiH in dehalogenation reactions. A few examples are given in Reactions (4.68) and (4.69). The repetitive hydrogen transfer from the same molecule of H(RSiH) nH allows these compounds to be used in small quantities. Work up can be done by adding n-pentane and filtering off the polymeric material, which precipitates from the crude mixture, and in a few cases the isolation of the product was readily obtained. This approach has also been used as a procedure for the partial or total functionalization of the SiH bond (see Section 8.3). O O O O I O O Cl O Cl H R Si H H n AIBN, 80 �C R = n-C 6 H 13 R = Ph H R Si H H n AIBN, 80 �C R = n-C 6H 13 R = Ph O O O O 91% 98% O O 92% 98% O Cl (4.68) (4.69)
- Page 31 and 32: Bond Dissociation Enthalpies 23 2.2
- Page 33 and 34: Ion Thermochemistry 25 Table 2.4 Re
- Page 35 and 36: Ion Thermochemistry 27 Table 2.5 El
- Page 37 and 38: References 29 3. Goumri, A., Yuan,
- Page 39 and 40: 32 Hydrogen Donor Abilities of Sili
- Page 41 and 42: 34 Hydrogen Donor Abilities of Sili
- Page 43 and 44: 36 Hydrogen Donor Abilities of Sili
- Page 45 and 46: 38 Hydrogen Donor Abilities of Sili
- Page 47 and 48: 40 Hydrogen Donor Abilities of Sili
- Page 49 and 50: 42 Hydrogen Donor Abilities of Sili
- Page 51 and 52: 44 Hydrogen Donor Abilities of Sili
- Page 53 and 54: 46 Hydrogen Donor Abilities of Sili
- Page 55 and 56: 4 Reducing Agents 4.1 GENERAL ASPEC
- Page 57 and 58: General Aspects of Radical Chain Re
- Page 59 and 60: Tris(trimethylsilyl)silane 53 Therm
- Page 61 and 62: Tris(trimethylsilyl)silane 55 4.3.1
- Page 63 and 64: Tris(trimethylsilyl)silane 57 A goo
- Page 65 and 66: Tris(trimethylsilyl)silane 59 C(O)C
- Page 67 and 68: Tris(trimethylsilyl)silane 61 radic
- Page 69 and 70: Tris(trimethylsilyl)silane 63 86% y
- Page 71 and 72: Tris(trimethylsilyl)silane 65 RO RO
- Page 73 and 74: Tris(trimethylsilyl)silane 67 O AcO
- Page 75 and 76: Tris(trimethylsilyl)silane 69 Tris(
- Page 77 and 78: Other Silicon Hydrides 71 The reduc
- Page 79 and 80: Other Silicon Hydrides 73 The decre
- Page 81: Other Silicon Hydrides 75 Ph MeS O
- Page 85 and 86: Silicon Hydride / Thiol Mixture 79
- Page 87 and 88: Silylated Cyclohexadienes 81 and (4
- Page 89 and 90: References 83 34. Kawashima, E., Uc
- Page 91 and 92: References 85 104. Gimisis, T., Bal
- Page 93 and 94: 88 Addition to Unsaturated Bonds te
- Page 95 and 96: 90 Addition to Unsaturated Bonds ap
- Page 97 and 98: 92 Addition to Unsaturated Bonds 5.
- Page 99 and 100: 94 Addition to Unsaturated Bonds R
- Page 101 and 102: 96 Addition to Unsaturated Bonds Ph
- Page 103 and 104: 98 Addition to Unsaturated Bonds EP
- Page 105 and 106: 100 Addition to Unsaturated Bonds 5
- Page 107 and 108: 102 Addition to Unsaturated Bonds T
- Page 109 and 110: 104 Addition to Unsaturated Bonds R
- Page 111 and 112: 106 Addition to Unsaturated Bonds a
- Page 113 and 114: 108 Addition to Unsaturated Bonds 5
- Page 115 and 116: 110 Addition to Unsaturated Bonds S
- Page 117 and 118: 112 Addition to Unsaturated Bonds T
- Page 119 and 120: 114 Addition to Unsaturated Bonds I
- Page 121 and 122: 116 Addition to Unsaturated Bonds 8
- Page 123 and 124: 118 Addition to Unsaturated Bonds 7
- Page 125 and 126: 120 Unimolecular Reactions 1 Si(H)M
- Page 127 and 128: 122 Unimolecular Reactions t-Bu t-B
- Page 129 and 130: 124 Unimolecular Reactions MeO Si O
- Page 131 and 132: 126 Unimolecular Reactions t-Bu t-B
76 Reduc<strong>in</strong>g Agents<br />
RO<br />
O<br />
O<br />
O<br />
O<br />
RO OC(S)OPh<br />
S<br />
O<br />
O<br />
NH<br />
Ph<br />
O<br />
O<br />
H H<br />
Si<br />
Si<br />
H H<br />
AIBN, 80 �C<br />
Me H<br />
Si<br />
Si<br />
HMe<br />
AIBN, 80 �C<br />
4.4.3 SILYL SUBSTITUTED SILICON HYDRIDES<br />
RO<br />
O<br />
O<br />
RO<br />
O<br />
96%<br />
O<br />
87%<br />
O<br />
O<br />
O<br />
(4.64)<br />
(4.65)<br />
Follow<strong>in</strong>g the success <strong>of</strong> (TMS) 3SiH, a systematic <strong>in</strong>vestigation <strong>of</strong> organosilanes<br />
hav<strong>in</strong>g different silyl substituents at the SiH moiety has been carried out<br />
with the aim <strong>of</strong> discover<strong>in</strong>g other reagents and test<strong>in</strong>g how to tune the reactivity<br />
by the choice <strong>of</strong> substituents. The idea that they might be capable <strong>of</strong> susta<strong>in</strong><strong>in</strong>g<br />
analogous radical cha<strong>in</strong> reactions turned out to be correct. <strong>In</strong>deed, the reductions<br />
<strong>of</strong> organic chlorides, bromides, iodides, phenyl selenides, isonitriles and<br />
thionocarbonates by several other silyl substituted silicon hydrides have<br />
emerged.<br />
By replac<strong>in</strong>g one alkyl group by a R3Si group <strong>in</strong> trialkylsilanes the effect on<br />
the hydrogen donat<strong>in</strong>g ability <strong>of</strong> SiH moiety <strong>in</strong>creases by about one order <strong>of</strong><br />
magnitude, the effect be<strong>in</strong>g cumulative (see Chapter 3). Reductions <strong>of</strong> a variety<br />
<strong>of</strong> organic derivatives were carried out by us<strong>in</strong>g RSi(H)Me2, where R ¼ SiMe3,<br />
SiMe2SiMe3, or Si(SiMe3) 3, and the <strong>in</strong>itiation given by benzoyl peroxide or tertbutyl<br />
perbenzoate (AIBN was found not to be efficient <strong>in</strong> these cases) [106]. An<br />
example for the simplest silane is reported <strong>in</strong> Reaction (4.66). A few rate<br />
constants for the halogen abstraction by Me3SiSi(:)Me2 radicals are also<br />
known and are smaller than that <strong>of</strong> Et3Si: or higher than that <strong>of</strong> (TMS) 3Si:<br />
(Table 4.6).<br />
Cl Me3SiSi(H)Me2 PhC(O)OOBu-t<br />
O 110 �C<br />
O<br />
83%<br />
(4.66)