"Front Matter". In: Organosilanes in Radical Chemistry - Index of
"Front Matter". In: Organosilanes in Radical Chemistry - Index of
"Front Matter". In: Organosilanes in Radical Chemistry - Index of
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
72 Reduc<strong>in</strong>g Agents<br />
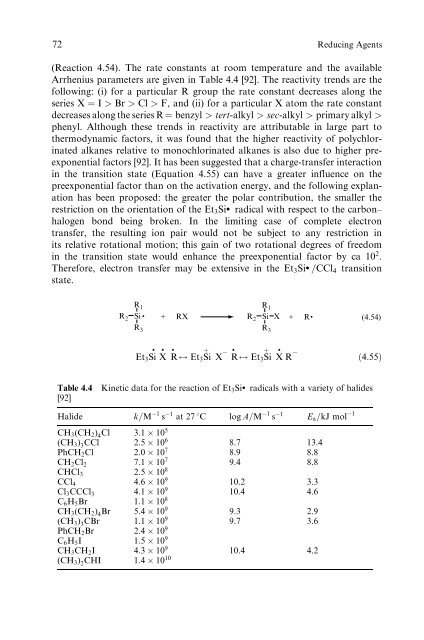
(Reaction 4.54). The rate constants at room temperature and the available<br />
Arrhenius parameters are given <strong>in</strong> Table 4.4 [92]. The reactivity trends are the<br />
follow<strong>in</strong>g: (i) for a particular R group the rate constant decreases along the<br />
series X ¼ I > Br > Cl > F, and (ii) for a particular X atom the rate constant<br />
decreases along the series R¼ benzyl > tert-alkyl > sec-alkyl > primary alkyl ><br />
phenyl. Although these trends <strong>in</strong> reactivity are attributable <strong>in</strong> large part to<br />
thermodynamic factors, it was found that the higher reactivity <strong>of</strong> polychlor<strong>in</strong>ated<br />
alkanes relative to monochlor<strong>in</strong>ated alkanes is also due to higher preexponential<br />
factors [92]. It has been suggested that a charge-transfer <strong>in</strong>teraction<br />
<strong>in</strong> the transition state (Equation 4.55) can have a greater <strong>in</strong>fluence on the<br />
preexponential factor than on the activation energy, and the follow<strong>in</strong>g explanation<br />
has been proposed: the greater the polar contribution, the smaller the<br />
restriction on the orientation <strong>of</strong> the Et3Si: radical with respect to the carbon–<br />
halogen bond be<strong>in</strong>g broken. <strong>In</strong> the limit<strong>in</strong>g case <strong>of</strong> complete electron<br />
transfer, the result<strong>in</strong>g ion pair would not be subject to any restriction <strong>in</strong><br />
its relative rotational motion; this ga<strong>in</strong> <strong>of</strong> two rotational degrees <strong>of</strong> freedom<br />
<strong>in</strong> the transition state would enhance the preexponential factor by ca 10 2 .<br />
Therefore, electron transfer may be extensive <strong>in</strong> the Et3Si:=CCl4 transition<br />
state.<br />
R 2<br />
R1 Si<br />
R3 + RX R2 R1 Si<br />
R3 X + R<br />
(4.54)<br />
Et3S : iX : R : $ Et3Si<br />
þ<br />
X R : $ Et3Si<br />
þ<br />
X : R ð4:55Þ<br />
Table 4.4 K<strong>in</strong>etic data for the reaction <strong>of</strong> Et3Si: radicals with a variety <strong>of</strong> halides<br />
[92]<br />
Halide k=M 1 s 1 at 27 8C log A=M 1 s 1 Ea=kJ mol 1<br />
CH3(CH2) 4Cl 3:1 105 (CH3) 3CCl 2:5 106 PhCH2Cl 2:0 10<br />
8.7 13.4<br />
7 CH2Cl2 7:1 10<br />
8.9 8.8<br />
7 CHCl3 2:5 10<br />
9.4 8.8<br />
8<br />
CCl4 4:6 109 Cl3CCCl3 4:1 10<br />
10.2 3.3<br />
9 C6H5Br 1:1 10<br />
10.4 4.6<br />
8<br />
CH3(CH2) 4Br 5:4 109 (CH3) 3CBr 1:1 10<br />
9.3 2.9<br />
9 PhCH2Br 2:4 10<br />
9.7 3.6<br />
9<br />
C6H5I 1:5109 CH3CH2I 4:3109 (CH3) 2CHI 1:4 10<br />
10.4 4.2<br />
10