"Front Matter". In: Organosilanes in Radical Chemistry - Index of
"Front Matter". In: Organosilanes in Radical Chemistry - Index of
"Front Matter". In: Organosilanes in Radical Chemistry - Index of
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Oxygen-Centred <strong>Radical</strong>s 41<br />
<strong>in</strong> determ<strong>in</strong><strong>in</strong>g the t-BuO: radical–silane reactivity. The trends outl<strong>in</strong>ed above<br />
can be entirely attributed to more favorable thermodynamic factors along the<br />
series.<br />
3.3.2 PEROXYL RADICALS<br />
The k<strong>in</strong>etics for the reaction <strong>of</strong> cumylperoxyl radical with a variety <strong>of</strong> silanes<br />
(Reaction 3.15) have been measured by us<strong>in</strong>g <strong>in</strong>hibited hydrocarbon oxidation<br />
methodology (Table 3.4) [29]. The trends <strong>in</strong> reactivity for the cumylperoxyl<br />
radical are similar to those observed for t-BuO: radical, although the reactions<br />
are about seven orders <strong>of</strong> magnitude slower. The rate constants <strong>in</strong>crease along<br />
the two series PhSi(H)Me2 < Ph2Si(H)Me < Ph3SiH and PhSiH3 < Ph2SiH2<br />
< Ph3SiH, when the statistical number <strong>of</strong> abstracted hydrogens is taken <strong>in</strong>to<br />
account. Furthermore, the rate constants <strong>in</strong>crease about 7 and 660 times on<br />
go<strong>in</strong>g from t-BuMe2SiH to Ph3SiH and (Me3Si) 3SiH, respectively.<br />
PhMe2COO: þ R3SiH !PhMe2COOH þ R3Si: (3:15)<br />
The similar reactivity <strong>of</strong> peroxyl with (CF3) 2NO: radicals towards a large<br />
variety <strong>of</strong> substrates has been observed previously and attributed to rather<br />
similar thermochemistries and sp<strong>in</strong> density distributions for these two radicals<br />
[30]. The Arrhenius parameters for the reaction <strong>of</strong> the persistent (CF3) 2NO:<br />
radical with n-Bu3SiH determ<strong>in</strong>ed by k<strong>in</strong>etic EPR spectroscopy are<br />
log A=M 1 s 1 ¼ 5:5 and Ea ¼ 32:2 kJ/mol, which corresponds to<br />
k ¼ 4:3M 1 s 1 at 73 8C [31].<br />
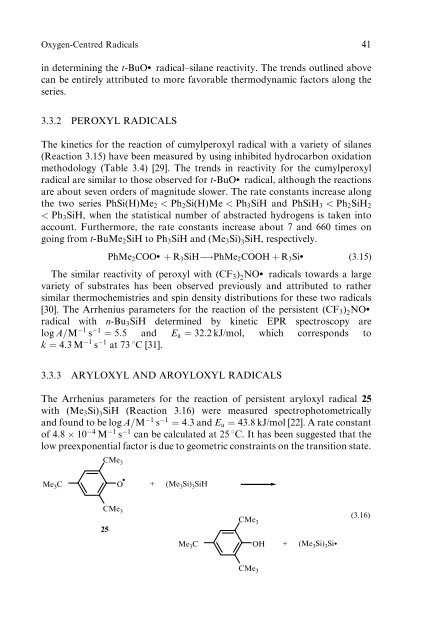
3.3.3 ARYLOXYL AND AROYLOXYL RADICALS<br />
The Arrhenius parameters for the reaction <strong>of</strong> persistent aryloxyl radical 25<br />
with (Me3Si) 3SiH (Reaction 3.16) were measured spectrophotometrically<br />
and found to be log A=M 1 s 1 ¼ 4:3 and Ea ¼ 43:8 kJ/mol [22]. A rate constant<br />
<strong>of</strong> 4:8 10 4 M 1 s 1 can be calculated at 25 8C. It has been suggested that the<br />
low preexponential factor is due to geometric constra<strong>in</strong>ts on the transition state.<br />
Me 3 C<br />
CMe 3<br />
CMe 3<br />
25<br />
O + (Me3Si) 3SiH Me 3 C<br />
CMe 3<br />
CMe 3<br />
OH + (Me3Si) 3Si (3.16)