"Front Matter". In: Organosilanes in Radical Chemistry - Index of
"Front Matter". In: Organosilanes in Radical Chemistry - Index of
"Front Matter". In: Organosilanes in Radical Chemistry - Index of
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Silylated Fullerenes 199<br />
EPR studies showed that R3Si: radicals (R ¼ Me, Et, i-Pr and t-Bu),<br />
obta<strong>in</strong>ed by hydrogen abstraction from the parent silanes by photogenerated<br />
t-BuO: radicals, add to C60 to form the correspond<strong>in</strong>g adducts [29]. The spectra<br />
<strong>of</strong> Me3Si- and t-Bu3Si-adducts have hyperf<strong>in</strong>e structure due to 9 and 27 equivalent<br />
protons, respectively, at 27 8C suggest<strong>in</strong>g free rotation <strong>of</strong> Si w C bonds on<br />
the EPR time scale. On the other hand, the middle members <strong>of</strong> the series, Et3Siand<br />
i-Pr3Si-adduct radicals, showed a unexpected hyperf<strong>in</strong>e manifold which has<br />
been accommodated with free rotation about the Si w C60 and frozen rotation<br />
about the Si w R bonds on the EPR time scale.<br />
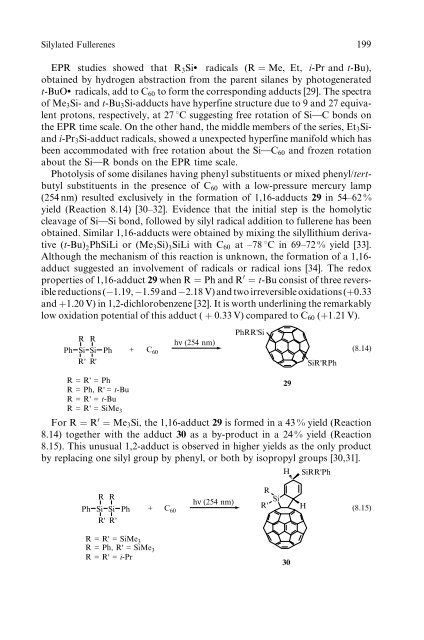
Photolysis <strong>of</strong> some disilanes hav<strong>in</strong>g phenyl substituents or mixed phenyl/tertbutyl<br />
substituents <strong>in</strong> the presence <strong>of</strong> C60 with a low-pressure mercury lamp<br />
(254 nm) resulted exclusively <strong>in</strong> the formation <strong>of</strong> 1,16-adducts 29 <strong>in</strong> 54–62 %<br />
yield (Reaction 8.14) [30–32]. Evidence that the <strong>in</strong>itial step is the homolytic<br />
cleavage <strong>of</strong> Si w Si bond, followed by silyl radical addition to fullerene has been<br />
obta<strong>in</strong>ed. Similar 1,16-adducts were obta<strong>in</strong>ed by mix<strong>in</strong>g the silyllithium derivative<br />
(t-Bu) 2PhSiLi or (Me3Si) 3SiLi with C60 at –78 8C <strong>in</strong> 69–72 % yield [33].<br />
Although the mechanism <strong>of</strong> this reaction is unknown, the formation <strong>of</strong> a 1,16adduct<br />
suggested an <strong>in</strong>volvement <strong>of</strong> radicals or radical ions [34]. The redox<br />
properties <strong>of</strong> 1,16-adduct 29 when R ¼ Ph and R 0 ¼ t-Bu consist <strong>of</strong> three reversible<br />
reductions ( 1.19, 1.59 and 2.18 V) and two irreversible oxidations (þ0.33<br />
and þ1.20 V) <strong>in</strong> 1,2-dichlorobenzene [32]. It is worth underl<strong>in</strong><strong>in</strong>g the remarkably<br />
low oxidation potential <strong>of</strong> this adduct ( þ 0:33 V) compared to C60 (þ1:21 V).<br />
R R<br />
Ph Si Si Ph<br />
R' R'<br />
+<br />
C 60<br />
hν (254 nm)<br />
PhRR'Si<br />
SiR'RPh<br />
(8.14)<br />
R = R' = Ph<br />
29<br />
R = Ph, R' = t-Bu<br />
R = R' = t-Bu<br />
R = R' = SiMe3 For R ¼ R0 ¼ Me3Si, the 1,16-adduct 29 is formed <strong>in</strong> a 43 % yield (Reaction<br />
8.14) together with the adduct 30 as a by-product <strong>in</strong> a 24 % yield (Reaction<br />
8.15). This unusual 1,2-adduct is observed <strong>in</strong> higher yields as the only product<br />
by replac<strong>in</strong>g one silyl group by phenyl, or both by isopropyl groups [30,31].<br />
R R<br />
Ph Si Si Ph<br />
R' R'<br />
+<br />
R = R' = SiMe 3<br />
R = Ph, R' = SiMe 3<br />
R = R' = i-Pr<br />
C 60<br />
hν (254 nm)<br />
H SiRR'Ph<br />
R<br />
R'<br />
Si<br />
H<br />
30<br />
(8.15)