"Front Matter". In: Organosilanes in Radical Chemistry - Index of
"Front Matter". In: Organosilanes in Radical Chemistry - Index of
"Front Matter". In: Organosilanes in Radical Chemistry - Index of
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
154 Consecutive <strong>Radical</strong> Reactions<br />
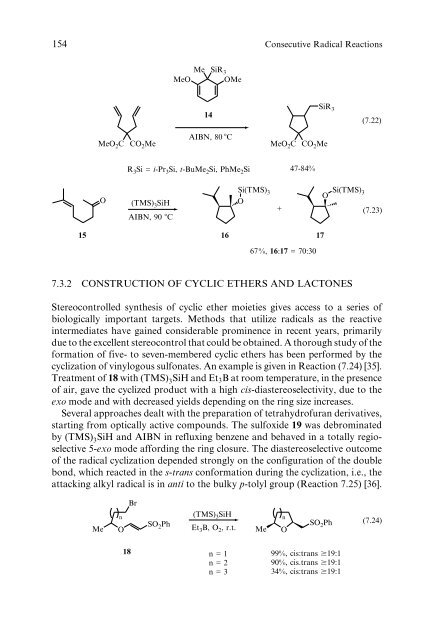
MeO 2 C CO 2 Me<br />
Me SiR 3<br />
MeO OMe<br />
14<br />
AIBN, 80 �C<br />
R 3 Si = i-Pr 3 Si, t-BuMe 2 Si, PhMe 2 Si<br />
O (TMS) 3SiH Si(TMS) 3<br />
O<br />
AIBN, 90 �C<br />
SiR 3<br />
MeO 2 C CO 2 Me<br />
+<br />
47-84%<br />
15 16 17<br />
67%, 16:17 = 70:30<br />
O Si(TMS) 3<br />
7.3.2 CONSTRUCTION OF CYCLIC ETHERS AND LACTONES<br />
(7.22)<br />
(7.23)<br />
Stereocontrolled synthesis <strong>of</strong> cyclic ether moieties gives access to a series <strong>of</strong><br />
biologically important targets. Methods that utilize radicals as the reactive<br />
<strong>in</strong>termediates have ga<strong>in</strong>ed considerable prom<strong>in</strong>ence <strong>in</strong> recent years, primarily<br />
due to the excellent stereocontrol that could be obta<strong>in</strong>ed. A thorough study <strong>of</strong> the<br />
formation <strong>of</strong> five- to seven-membered cyclic ethers has been performed by the<br />
cyclization <strong>of</strong> v<strong>in</strong>ylogous sulfonates. An example is given <strong>in</strong> Reaction (7.24) [35].<br />
Treatment <strong>of</strong> 18 with (TMS) 3SiH and Et3B at room temperature, <strong>in</strong> the presence<br />
<strong>of</strong> air, gave the cyclized product with a high cis-diastereoselectivity, due to the<br />
exo mode and with decreased yields depend<strong>in</strong>g on the r<strong>in</strong>g size <strong>in</strong>creases.<br />
Several approaches dealt with the preparation <strong>of</strong> tetrahydr<strong>of</strong>uran derivatives,<br />
start<strong>in</strong>g from optically active compounds. The sulfoxide 19 was debrom<strong>in</strong>ated<br />
by (TMS) 3SiH and AIBN <strong>in</strong> reflux<strong>in</strong>g benzene and behaved <strong>in</strong> a totally regioselective<br />
5-exo mode afford<strong>in</strong>g the r<strong>in</strong>g closure. The diastereoselective outcome<br />
<strong>of</strong> the radical cyclization depended strongly on the configuration <strong>of</strong> the double<br />
bond, which reacted <strong>in</strong> the s-trans conformation dur<strong>in</strong>g the cyclization, i.e., the<br />
attack<strong>in</strong>g alkyl radical is <strong>in</strong> anti to the bulky p-tolyl group (Reaction 7.25) [36].<br />
Me<br />
Br<br />
n<br />
O<br />
SO2Ph (TMS) 3SiH<br />
Et3B, O2, r.t. Me<br />
n<br />
O<br />
18 n = 1<br />
n = 2<br />
n = 3<br />
SO 2 Ph<br />
99%, cis:trans �19:1<br />
90%, cis.trans �19:1<br />
34%, cis:trans �19:1<br />
(7.24)