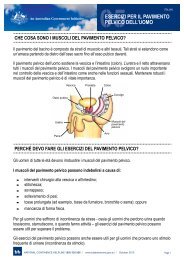
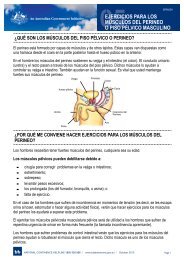
Pharmacy Continence Care - Bladder and Bowel Website
Pharmacy Continence Care - Bladder and Bowel Website
Pharmacy Continence Care - Bladder and Bowel Website
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Two instruments are widely used for the measurement of consumer-reported incontinence<br />
<strong>and</strong> continence experience <strong>and</strong> impacts (social, physical <strong>and</strong> financial). The more extensive<br />
instrument, the Dowell Bryant Incontinence Cost Index (DBICI), was not considered suitable<br />
to administer, given time <strong>and</strong> cost limitations for the PCCP, as it is an extensive economic<br />
questionnaire that has been designed to be administered by nurse continence advisers. The<br />
Incontinence Impact Questionnaire (IIQ), which has been internationally <strong>and</strong> locally<br />
validated <strong>and</strong> which has both a long <strong>and</strong> short form, was determined, in consultation with<br />
the NOVA Expert Panel, to be the preferred instrument to measure changes in reported<br />
health <strong>and</strong> wellbeing outcomes for people with or at risk of incontinence. The project team<br />
used the IIQ question format to include assessment of carer perceptions of the social,<br />
physical <strong>and</strong> financial impacts for them of the incontinence of the person receiving care.<br />
To evaluate pharmacy benefits:<br />
• NOVA team members designed structured interview schedules for telephone<br />
administration with nominated personnel in each participating pharmacy. The first<br />
interview schedule, the <strong>Pharmacy</strong> Intake Questionnaire – PIQ, was administered by<br />
telephone at the inception of the Program, before the training provided in each<br />
pharmacy, <strong>and</strong> the second interview schedule, the <strong>Pharmacy</strong> Exit Questionnaire – PEQ,<br />
was administered by telephone at the conclusion of the pilot Program trial period in each<br />
pharmacy.<br />
• The administration of the training program was evaluated through completion by<br />
participating pharmacies of a self-administered questionnaire providing feedback on the<br />
training experience <strong>and</strong> outcomes. To promote return of these questionnaires,<br />
participating pharmacies were offered an incentive payment of $350, on receipt of the<br />
returned questionnaire, to defray costs to the pharmacy of participation in the training.<br />
To evaluate consumer benefits:<br />
• The training program <strong>and</strong> resources provided each participating pharmacy with<br />
information <strong>and</strong> guidance in identifying, by both observation <strong>and</strong> by medication<br />
dispensed, customers who potentially could be at risk of, or are not coping with,<br />
incontinence. <strong>Pharmacy</strong> staff were provided with information <strong>and</strong> advice through the<br />
training program on approaching customers <strong>and</strong> offering continence information.<br />
Through the training <strong>and</strong> the follow-up support by the project team, pharmacy staff were<br />
also encouraged to recruit customers who had responded to the continence care service<br />
to complete a research questionnaire whilst they were in the pharmacy.<br />
• Market research firm Ipsos Australia Pty Ltd was sub-contracted by NOVA Public Policy to<br />
undertake the consumer survey component of the project. NOVA <strong>and</strong> IPSOS developed a<br />
baseline survey questionnaire for completion in-pharmacy by customers with whom a<br />
pharmacy staff member had discussed incontinence (the intervention). The consumer<br />
baseline study was designed to establish consumers' awareness of continence<br />
management, their experience of the social, financial <strong>and</strong> physical impact of<br />
incontinence, using the IIQ, <strong>and</strong> their past or present use of other health services for<br />
incontinence. To measure benefit, the questionnaire was adjusted to be administered<br />
post-intervention to respondents who consented to follow-up, at three months after the<br />
baseline questionnaire, through computer assisted telephone interview (CATI).<br />
Element 5 – Develop Draft St<strong>and</strong>ards<br />
The project team was to develop st<strong>and</strong>ards for potential incorporation into the QCPP<br />
program. The project results made clear, however, that the development of separate<br />
st<strong>and</strong>ards was neither appropriate nor desirable for the project. Consequently, an alternative<br />
Final Report<br />
9<br />
NOVA Public Policy<br />
<strong>Pharmacy</strong> <strong>Continence</strong> <strong>Care</strong> Project