Prader-Willi Syndrome: Genetic Tests and Clinical ... - Genoma - USP
Prader-Willi Syndrome: Genetic Tests and Clinical ... - Genoma - USP
Prader-Willi Syndrome: Genetic Tests and Clinical ... - Genoma - USP
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
GENETIC TESTING<br />
Volume 4, Number 4, 2000<br />
Mary Ann Liebert, Inc.<br />
<strong>Prader</strong>-<strong>Willi</strong> <strong>Syndrome</strong>: <strong>Genetic</strong> <strong>Tests</strong> <strong>and</strong> <strong>Clinical</strong> Findings<br />
CINTIA FRIDMAN, 1 MONICA C. VARELA, 1 FERNANDO KOK, 2 NUVARTE SETIAN, 3<br />
<strong>and</strong> CÉLIA P. KOIFFMANN 1<br />
ABSTRACT<br />
Here we describe the genetic studies performed in 53 patients with the suspected diagnosis of <strong>Prader</strong>-<strong>Willi</strong><br />
syndrome (PWS). PWS is characterized by neonatal hypotonia, hypogonadism, delayed psychomotor development,<br />
hyperphagia, obesity, short stature, small h<strong>and</strong>s <strong>and</strong> feet, learning disabilities, <strong>and</strong> obsessive-compulsive<br />
behavior. Through the methylation analysis of the SNRPN gene, microsatellite studies of loci mapped<br />
within <strong>and</strong> outside the PWS/AS region, <strong>and</strong> fluorescence in situ hybridization (FISH) study, we confirmed the<br />
diagnosis in 35 patients: 27 with a paternal deletion, <strong>and</strong> 8 with maternal uniparental disomy (UPD). The clinical<br />
comparisons between deleted <strong>and</strong> UPD patients indicated that there were no major phenotype differences,<br />
except for a lower birth length observed in the UPD children. Our sample was composed of more girls than<br />
boys; UPD patients were diagnosed earlier than the deleted cohort (2 10/12 vs. 7 9/12 years); <strong>and</strong>, in the deleted<br />
group, the boys were diagnosed earlier than the girls (5 2/12 vs. 7 8/12 years, respectively).<br />
INTRODUCTION<br />
PRADER-WILLI SYNDROME (PWS) is a disease that occurs with<br />
an incidence of , 1:15–20,000 births, <strong>and</strong> is associated with<br />
developmental <strong>and</strong> behavioral problems. PWS children show<br />
neonatal hypotonia, hypogonadism, failure to thrive, hyperphagia,<br />
obesity, short stature, small h<strong>and</strong>s <strong>and</strong> feet, mental retardation<br />
with learning disability, <strong>and</strong> obsessive-compulsive behavior<br />
(<strong>Prader</strong> et al., 1956; Cassidy, 1997).<br />
Different mechanisms can lead to PWS, which is due to a<br />
common genetic deficit (Glenn et al., 1997) that abolishes the<br />
expression of imprinted paternal genes. The major genetic<br />
mechanism giving rise to PWS is a paternal deletion of about<br />
the same size in the 15q11–q13 region, that occurs in , 70%<br />
of the cases. Maternal uniparental disomy (UPD) is the second<br />
mechanism, occurring in , 25% of affected individuals. Because<br />
the presence of two complete maternal chromosomes 15<br />
cannot substitute for the absence of paternal alleles, it is suggested<br />
that the 15q11–q13 region is imprinted (Nicholls et al.,<br />
1989; Glenn et al., 1997). About 1–5% of patients with PWS<br />
have biparental inheritance of chromosome 15, but show abnormal<br />
methylation patterns <strong>and</strong> gene expression. These pa-<br />
387<br />
tients have a mutation in the imprinting process (Buiting et al.,<br />
1995; Dittrich et al., 1996; Saitoh et al., 1996, 1997; Ohta et<br />
al., 1999).<br />
The test of the methylation pattern of SNRPN exon 1 detects<br />
95% of the PWS <strong>and</strong> 80% of the Angelman <strong>Syndrome</strong> (AS) patients,<br />
<strong>and</strong> the microsatellite analysis of loci within <strong>and</strong> outside<br />
the PWS/AS region distinguishes between the genetic mechanisms<br />
in each case (deletion, UPD, or an imprinting mutation).<br />
G-b<strong>and</strong> analysis is recommended to distinguish numerical <strong>and</strong>/or<br />
structural chromosome aberrations, such as translocations, inversions,<br />
or marker chromosomes; fluorescence in situ hybridization<br />
(FISH) techniques are useful in detecting the deletion<br />
cases, when parental DNA samples are not available, <strong>and</strong><br />
also to identify the specific chromosome aberrations detected.<br />
Some genes (SNURF-SNRPN, IPW, ZNF127, NECDIN) <strong>and</strong><br />
transcripts (PAR1 <strong>and</strong> PAR5) are paternally expressed <strong>and</strong> map<br />
to the PWS/AS critical region. Therefore, they are good c<strong>and</strong>idates<br />
to be involved in the etiology of PWS (Glenn et al., 1997;<br />
MacDonald <strong>and</strong> Wevrick, 1997; Saitoh et al., 1996). However,<br />
only two of them (SNRPN <strong>and</strong> NECDIN) have a known protein<br />
that is completely absent in PWS patients. The SNRPN protein<br />
(SmN) is involved in RNA splicing, whereas NECDIN is<br />
1 Department of Biology, Institute of Bioscience, University of São Paulo, São Paulo, Brazil.<br />
2 Child Neurology Service, Department of Neurology, Hospital das Clínicas, School of Medicine, University of São Paulo, São Paulo, Brazil.<br />
3 Pediatric Endocrinology Unit, Department of Pediatrics, Hospital das Clínicas, School of Medicine, University of São Paulo, São Paulo,<br />
Brazil.
388<br />
a nuclear protein that acts, mainly, in cerebral development<br />
(Glenn et al., 1996; Jay et al., 1997). These genes show paternal<br />
expression in humans <strong>and</strong> mice, <strong>and</strong> map to the PWS/AS<br />
critical region in humans <strong>and</strong> to the syntenic conserved region<br />
in the central part of chromosome 7 in mice (Glenn et al., 1996;<br />
Jay et al., 1997; MacDonald <strong>and</strong> Wevrick, 1997; Watrin et al.,<br />
1997; Gray et al., 1999).<br />
PWS is presumed to be a contiguous gene disease because<br />
no patients with mutations in a single gene have so far been detected.<br />
Phenotype characterization is, therefore, important to underst<strong>and</strong><br />
the possible roles of genes in the critical region, with<br />
manifestations of the syndrome. Until now, two main clinical<br />
differences have been observed between the different classes of<br />
PWS patients: hypopigmentation, most common in deleted patients,<br />
is due to the loss of the P gene (Spritz et al., 1997),<br />
whereas increased maternal age has been associated with UPD<br />
cases (Butler, 1989; Gillessen-Kaesbach et al., 1995).<br />
In this report we studied PWS patients diagnosed through<br />
methylation analysis of the SNRPN exon 1, microsatellites, <strong>and</strong><br />
FISH. <strong>Clinical</strong> features of 27 deleted PWS patients are described<br />
<strong>and</strong> compared with those of 8 patients with maternal<br />
UPD.<br />
Patients<br />
MATERIALS AND METHODS<br />
Fifty-three patients suspected clinically of having PWS were<br />
referred to our laboratory for genetic tests. The majority of these<br />
patients were seen by at least one of us, but in some cases only<br />
a blood sample was sent to us, with a short list of clinical characteristics.<br />
The main features that prompted physicians to ask<br />
for these studies were hypotonia in infants, <strong>and</strong> obesity <strong>and</strong><br />
mental retardation in children <strong>and</strong> adolescents.<br />
Methods<br />
Cytogenetic studies: Chromosome studies of patients <strong>and</strong><br />
their parents were performed on peripheral blood lymphocytes<br />
to investigate structural <strong>and</strong> numerical alterations. The chromosomes<br />
were examined by the GTG b<strong>and</strong>ing technique.<br />
FISH was performed using the SNRPN probe, specific for<br />
the 15q11–q13 region, <strong>and</strong> control chromosome 15 marker cosmids,<br />
which detect specific sequences in 15q22. The hybridization<br />
<strong>and</strong> immunochemical detection were carried out according<br />
to the manufacturer’s instructions (Oncor, Inc.). Twenty<br />
cells were examined for each FISH slide.<br />
DNA analysis: Methylation analysis. DNA was extracted<br />
from peripheral blood leukocytes by st<strong>and</strong>ard procedures. The<br />
methylation status of the PWS/AS region was assessed by<br />
Southern blotting (Southern, 1975). Genomic DNA was digested<br />
with XbaI 1 NotI, separated by size on a 1.0% agarose<br />
gel, transferred to a nylon membrane, <strong>and</strong> hybridized using the<br />
probe that corresponds to a 0.6-kb EcoRI–NotI fragment that<br />
contains exon 1 of SNRPN (Glenn et al., 1996).<br />
Dinucleotide repeat (CA) n polymorphisms within the<br />
PWS/AS critical region. Microsatellite analysis was performed<br />
with three markers within the critical region, 15q11–q13, 4-<br />
3RCA (D15S11); LS6-1CA (D15S113); <strong>and</strong> GABRB3CA<br />
(GABRB3). Two loci outside the PWS/AS region, D15S131 <strong>and</strong><br />
D15S984, were also studied to distinguish between deletion <strong>and</strong><br />
UPD. Deletion is suggested if there is biparental inheritance of<br />
these loci; isodisomy, if one maternal allele is present; <strong>and</strong> heterodisomy,<br />
if two different maternal alleles are evident. For<br />
UPD patients, we also analyzed the loci D15S41 <strong>and</strong> D15S42,<br />
localized close to the centromere, making it possible to identify<br />
the meiotic origin of the non-disjunction (Robinson et al.,<br />
1998). Multiplex PCR <strong>and</strong> polyacrylamide gel electrophoresis<br />
of 32 P-end-labeled amplification products followed the protocols<br />
described by Mutirangura et al. (1993).<br />
Statistical analysis<br />
Statistical analysis was performed using the Fisher test, Student’s<br />
t-test, <strong>and</strong> Mann-Whitney’s test. Confidence levels of<br />
a 5 0.05 were used to indicate a statistically significant difference<br />
between the different classes of PWS patients (with<br />
deletion or UPD).<br />
RESULTS<br />
Of 53 patients suspected of having PWS who were referred<br />
for genetic tests, the syndrome was confirmed in 35 (20 girls<br />
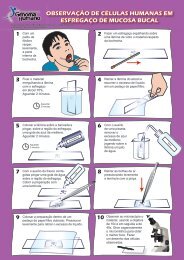
<strong>and</strong> 15 boys, with ages ranging from 3 months to 18 years) (Fig.<br />
1), through methylation pattern analysis.<br />
Microsatellite analysis of loci within <strong>and</strong> outside the<br />
PWS/AS region was performed in 30 of the 35 PWS patients,<br />
because parental samples were not available in 5 patients. We<br />
identified 18 patients with a paternal deletion, 8 with maternal<br />
UPD, <strong>and</strong> 4 patients with the absence of paternal alleles, but<br />
who were noninformative with regard to the specific genetic<br />
mechanism. In those 9 patients in which microsatellite studies<br />
were not available or informative, FISH showed a deletion at<br />
the PWS critical region. Seven cases with heterodisomies <strong>and</strong><br />
one with complete isodisomy were identified among the 8 UPD<br />
patients. In summary, we obtained 27 deletion cases (77.2%)<br />
<strong>and</strong> 8 maternal UPD patients (22.8%).<br />
The main clinical features of the UPD <strong>and</strong> deleted patients<br />
are summarized in Table 1.<br />
DISCUSSION<br />
FRIDMAN ET AL.<br />
In this study, we diagnosed 35 PWS cases in 53 patients referred<br />
for genetic evaluation. <strong>Clinical</strong> characteristics of 27 individuals<br />
with a paternal deletion (14 girls <strong>and</strong> 13 boys), <strong>and</strong> 8<br />
patients with maternal UPD (6 girls <strong>and</strong> 2 boys), were compared<br />
(Table 1).<br />
Previous clinical comparisons between deleted <strong>and</strong> UPD<br />
PWS patients pointed only to differences in pigmentation, with<br />
hypopigmentation more frequent in deleted patients, <strong>and</strong> in maternal<br />
age, which has been reported to be increased in the UPD<br />
patients (Butler, 1989; Robinson et al., 1991; Gillessen-Kaesbach<br />
et al., 1995). Nevertheless, in our data (Table 1) a significant<br />
difference in the age at diagnosis between the two classes<br />
of PWS patients was observed: our children with UPD were diagnosed<br />
earlier than the deleted ones (2 10/12 vs. 7 5/12 years, respectively),<br />
different from the studies of Mitchell et al. (1996)
PWS SYNDROME<br />
c<br />
a<br />
d e<br />
b<br />
f g h i j<br />
FIG. 1. <strong>Prader</strong>-<strong>Willi</strong> patients. (a) patient at 24/12 years; (b) patient at 15 years; (c) patient at 9 months; (d) patient at 118/12 years; (e) patient at 18 years; (f) patient at 29/12 years;<br />
(g) patient at 118/12 years; (h) patient at 5 years; (i) patient at 5 years; (j) patient at 10 months. Patients a, c, h, <strong>and</strong> i are UPD cases; patients b, d, e, f, g, <strong>and</strong> j are deletion cases.<br />
389
390<br />
TABLE 1. CLINICAL FINDINGS IN PWS PATIENTS WITH DELETION AND UPD<br />
<strong>and</strong> Gunay-Aygun et al. (1997a). Within the deleted group, the<br />
boys were diagnosed earlier than the girls (5 2/12 vs. 7 8/12 years,<br />
respectively).<br />
As expected, mean maternal age in the UPD group was<br />
higher than in the deleted group, because increased maternal<br />
age is associated with the meiotic non-disjunctive events that<br />
originate UPD zygotes (Robinson et al., 1993, 1998). Mean paternal<br />
age was also elevated in the UPD group; not unexpected<br />
because paternal age is usually related to maternal age.<br />
Only half of our UPD sample had hyperphagia <strong>and</strong> showed<br />
weights above the 97%. Children that were not in a hyperphagic<br />
phase had weights below the 25% <strong>and</strong> did not have the characteristic<br />
PWS behavioral features.<br />
PWS UPD patients showed lower birth lengths than the<br />
deleted patients, as observed in other studies (Mitchell et al.,<br />
1996; Gunay-Aygun et al., 1997a). In the 7 patients with heterodisomy,<br />
placental insufficiency due to the trisomy preceding<br />
maternal chromosome 15 UPD could explain such differences<br />
(Gunay-Aygun et al., 1997a). No differences in facial<br />
features, such as narrow bifrontal diameter, almond-shaped<br />
eyes, <strong>and</strong> strabismus, as suggested by Cassidy et al. (1997),<br />
were observed.<br />
Among patients with normal genetic results, obesity <strong>and</strong><br />
mental retardation in children <strong>and</strong> adolescents, <strong>and</strong> hypotonia<br />
FRIDMAN ET AL.<br />
UPD Deletion<br />
(8 patients) (27 patients) P a<br />
Mean age of diagnosis (y) (range) 2 10/12 7 9/12 0.0152 (1)<br />
(3/12 to 6) (9/12 to 18)<br />
Sex ratio (Female; Male) 6; 2 14; 13<br />
Mean maternal age (y) (range) 36 10/12 27 2/12 0.0022 (1)<br />
(19 to 47) (20 to 38)<br />
Mean paternal age (y) (range) 38 6/12 29 8/12 0.0088 (1)<br />
(21 to 50) (23 to 43)<br />
Reduced fetal activity 71,43% (5/7) 77,77% (14/18) 1.0000 (2)<br />
Birth weight (g) (%) 2521 (p3) 2693 (3,p,10) 0.3616 (3)<br />
Birth length (cm) (%) 44,42 (,,p3) 47,87 (p10) 0.0298 (3)<br />
Hypotonia 100% (8/8) 96% (24/25) 1.0000 (2)<br />
Feeding problems 100% (8/8) 96% (24/25) 1.0000 (2)<br />
Developmental delay 87,5% (7/8) 100% (26/26) 0.2353 (2)<br />
Weight .p90 50% (4/8) 73,08% (19/26) 0.3884 (2)<br />
Height ,p50 42,86% (3/7) 50% (12/24) 1.0000 (2)<br />
OFC ,p50 37,5% (3/8) 68,18% (15/22) 0.2098 (2)<br />
OFC 50,p,97 62,5% (5/8) 31,82% (7/22) 0.2098 (2)<br />
Hypertelorism 62,5% (5/8) 40% (8/20) 0.4097 (2)<br />
Almond shaped eyes 62,5% (5/8) 52,38% (11/21) 0.6968 (2)<br />
Strabismus 71,42% (5/7) 45,45% (10/22) 0.3898 (2)<br />
Narrow bifrontal diameter 50% (4/8) 86,36% (19/22) 0.0596 (2)<br />
Small h<strong>and</strong>s <strong>and</strong> feet 71,42% (5/7) 81,82% (18/22) 0.6119 (2)<br />
Learning disabilities 100% (1/1) 100% (16/16) 1.0000 (2)<br />
Seizures 16,67% (1/6) 54,54% (12/22) 0.1727 (2)<br />
Behavioral problems 80% (4/5) 78,94% (15/19) 1.0000 (2)<br />
Hyperphagia 50% (4/8) 72% (18/25) 0.3970 (2)<br />
High pain threshold 40% (2/5) 54,54% (6/11) 1.0000 (2)<br />
Skin picking 100% (2/2) 86,66% (13/15) 1.0000 (2)<br />
Decreased vomiting 100% (2/2) 100% (10/10) 1.0000 (2)<br />
a Statistical analysis with a level of confidence of a 5 0,05 to assume a statistically significance difference. (1) Mann-<br />
Whitney test; (2) Fisher test; (3) Student’s t-test.<br />
in babies were the main characteristics considered by the physicians<br />
in asking for PWS genetic tests.<br />
Some individuals with the PWS-like phenotype but with normal<br />
methylation results have been described in the medical literature,<br />
<strong>and</strong> hypotonia, obesity, mental retardation, <strong>and</strong> sometimes<br />
hyperphagia are the main observed characteristics of such<br />
cases. Additional investigation can disclose some other anomalies,<br />
like the Fragile X syndrome (de Vries et al., 1993), interstitial<br />
6q deletion (Villa et al., 1995; Stein et al., 1996),<br />
UPD14 (Berends et al., 1999), some chromosome X aberrations<br />
(Monaghan et al., 1998; Stratakis, 1998; Tümer et al., 1998),<br />
<strong>and</strong> 1p deletions (Eugster et al., 1997). Obesity <strong>and</strong> mental retardation<br />
can occur in many other mendelian inherited disorders,<br />
such as the Cohen syndrome, Bardet-Biedl syndrome, <strong>and</strong><br />
others (see review in Gunay-Aygun et al., 1997b).<br />
Besides obesity <strong>and</strong> mental retardation in children, a history<br />
of neonatal hypotonia <strong>and</strong> swallowing difficulties should be<br />
present to suggest PWS diagnosis. In babies, hypotonia, hypogonadism,<br />
feeding problems, <strong>and</strong> decreased vomiting suggest<br />
the diagnosis of the syndrome. The methylation analysis,<br />
a noninvasive exam, allows one to make the PWS diagnosis,<br />
<strong>and</strong> invasive exams, like muscle biopsy <strong>and</strong> electroneuromiography,<br />
which are usually performed in hypotonic babies, are not<br />
necessary. Consequently, with early PWS diagnosis, dietary
PWS SYNDROME<br />
management to avoid obesity <strong>and</strong> physiotherapy to improve<br />
muscle tone <strong>and</strong> avoid scoliosis, can be introduced earlier. PWS<br />
diagnosis in babies <strong>and</strong> children can avoid obesity-related health<br />
problems, which can cause early death. Parents <strong>and</strong> siblings of<br />
deletion <strong>and</strong> UPD PWS patients have low genetic risks (around<br />
1%), unless some type of chromosome aberration is disclosed<br />
in the patient. <strong>Clinical</strong> <strong>and</strong> behavioral PWS studies in populations<br />
of different genetic backgrounds are important to delineate<br />
the phenotypic <strong>and</strong> behavioral variability present in this<br />
syndrome.<br />
ACKNOWLEDGMENTS<br />
We thank Dr. Robert D. Nicholls for kindly provided the<br />
SNRPN probe for methylation assay. This work is supported by<br />
FAPESP <strong>and</strong> PRONEX.<br />
REFERENCES<br />
BERENDS, M.J.W., HORDIJK, R., SCHEFFER, H., OOSTERWIJK,<br />
J.C., HALLEY, D.J.J., <strong>and</strong> SORGEDRAGER, N. (1999). Two cases<br />
of maternal uniparental disomy 14 with a phenotype overlapping with<br />
the <strong>Prader</strong>-<strong>Willi</strong> phenotype. Am. J. Med. Genet. 84, 76–79.<br />
BUITING, K., SAITOH, S., GROSS, S., DITTRICH, B., SCHWARTZ,<br />
S., NICHOLLS, R.D., <strong>and</strong> HORSTHEMKE B. (1995). Inherited microdeletions<br />
in the Angelman <strong>and</strong> <strong>Prader</strong>-<strong>Willi</strong> syndromes define an<br />
imprinting center on human chromosome 15. Nature Genet 9,<br />
395–400.<br />
BUTLER, M.G. (1989). Hypopigmentation: a common feature of<br />
<strong>Prader</strong>-Labhart-<strong>Willi</strong> syndrome. Am. J. Hum. Genet. 45, 140–146.<br />
CASSIDY, S.B. (1997). <strong>Prader</strong>-<strong>Willi</strong> syndrome. J. Med. Genet. 34,<br />
917–923.<br />
CASSIDY, S.B., FORSYTHE, M., HEEGER, S., NICHOLLS, R.D.,<br />
SCHORK, N., BENN, P., <strong>and</strong> SCHWARTZ, S. (1997). Comparison<br />
of phenotype between patients with <strong>Prader</strong>-<strong>Willi</strong> syndrome due to<br />
deletion 15q <strong>and</strong> uniparental disomy 15. Am. J. Med. Genet. 68,<br />
433–440.<br />
DE BRIES, B.B.A., FRYNS, J-P., BUTLER, M.G., CANZIANI, F.,<br />
WESBY-VAN SWAAY, E., O VAN HEMEL, J., OOSTRA, B.A.,<br />
HALLEY, D.J.J., <strong>and</strong> NIERMEIJER, M.F. (1993). <strong>Clinical</strong> <strong>and</strong> molecular<br />
studies in fragile X patients with a <strong>Prader</strong>-<strong>Willi</strong>-like phenotype.<br />
J. Med. Genet. 30, 761–766.<br />
DITTRICH, B., BUITING, K., KORN, B., RICHARD, S., BUXTON,<br />
J., SAITOH, S., NICHOLLS, R.D., POUSTRA, A., WINTER-<br />
PACHT, A., ZABEL, B., <strong>and</strong> HORSTHEMKE, B. (1996). Imprinting<br />
switching on human chromosome 15 may involve alternative<br />
transcripts of the SNRPN gene. Nature Genet. 14, 163–170.<br />
EUGSTER, E.A., BERRY, S.A., <strong>and</strong> HIRSCH, B. (1997). Mosaicism<br />
for deletion 1p36.33 in a patient with obesity <strong>and</strong> hyperphagia. Am.<br />
J. Med. Genet. 70, 409–412.<br />
GILLESSEN-KAESBACH, G., ROBINSON, W.P., LOHMANN, D.,<br />
KAYA-WESTERLOH, S., PASSARGE, E., <strong>and</strong> HORSTHEMKE,<br />
B. (1995). Genotype-phenotype correlation in a series of 167 deletion<br />
<strong>and</strong> non-deletion patients with <strong>Prader</strong>-<strong>Willi</strong> syndrome. Hum.<br />
Genet. 96, 638–643.<br />
GLENN, C.C., SAITOH, S., JONG, M.T.C., FILBRANDT, M.M.,<br />
SURTI, U., DRISCOLL, D.J., <strong>and</strong> NICHOLLS, R.D. (1996). Gene<br />
structure, DNA methylation, <strong>and</strong> imprinted expression of the human<br />
SNRPN gene. Am. J. Hum. Genet. 58, 335–346.<br />
GLENN, C.C., DRISCOLL, D.J., YANG, T.P., <strong>and</strong> NICHOLLS, R.D.<br />
(1997). Genomic imprinting: potential function <strong>and</strong> mechanisms re-<br />
391<br />
vealed by the <strong>Prader</strong>-<strong>Willi</strong> <strong>and</strong> Angelman syndromes. Mol. Hum.<br />
Reprod. 3, 321–332.<br />
GRAY, T.A., SAITOH, S., <strong>and</strong> NICHOLLS, R.D. (1999). An imprinted,<br />
mammalian bicistronic transcript encodes two independent<br />
proteins. Proc. Natl. Acad. Sci. USA 96, 5616–5621.<br />
GUNAY-AYGUN, M., HEEGER, S., SCHWARTZ, S., <strong>and</strong> CAS-<br />
SIDY, S.B. (1997a). Delayed diagnosis in patients with <strong>Prader</strong>-<strong>Willi</strong><br />
syndrome due to maternal uniparental disomy 15. Am. J. Med. Genet.<br />
71, 106–110.<br />
GUNAY-AYGUN, M., CASSIDY, S.B., <strong>and</strong> NICHOLLS, R.D.<br />
(1997b). <strong>Prader</strong>-<strong>Willi</strong> <strong>and</strong> others syndromes associated with obesity<br />
<strong>and</strong> mental retardation. Behav. Genet. 27, 307–324.<br />
JAY, P., ROUGEULLE, C., MASSACRIER, A., MONCLA, A., MAT-<br />
TEI, M-G., MALZAC, P., ROËCKEL, N., TAVIAUX, S.,<br />
LEFRANC, J-L.B., CAU, P., BERTA, P., LALANDE, M., <strong>and</strong><br />
MUSCATELLI, F. (1997). The human necdin gene, NDN, is maternally<br />
imprinted <strong>and</strong> located in the <strong>Prader</strong>-<strong>Willi</strong> syndrome chromosomal<br />
region. Nature Genet. 17, 357–361.<br />
MACDONALD, H.R., <strong>and</strong> WEVRICK, R. (1997). The necdin gene is<br />
deleted in <strong>Prader</strong>-<strong>Willi</strong> syndrome <strong>and</strong> is imprinted in human <strong>and</strong><br />
mouse. Hum. Mol. Genet. 6, 1873–1878.<br />
MITCHELL, J., SCHINZEL, A., LANGLOIS, S., GILLESSEN-<br />
KAESBACH, G., SCHUFFENHAUER, S., MICHAELIS, R.C.,<br />
ABELIOVICH, D., LERER, I., CHRISTIAN, S., GUITART, M.,<br />
MCFADDEN, D.E., <strong>and</strong> ROBINSON, W.P. (1996). Comparison of<br />
phenotype in uniparental disomy <strong>and</strong> deletion <strong>Prader</strong>-<strong>Willi</strong> syndrome:<br />
sex specific differences. Am. J. Med. Genet. 65, 133–136.<br />
MONAGHAN, K.G., VAN DYKE, D.L., <strong>and</strong> FELDMANM, G.L.<br />
(1998). <strong>Prader</strong>-<strong>Willi</strong>-like syndrome in a patient with an Xq23q25 duplication.<br />
Am. J. Med. Genet. 80, 227–231.<br />
MUTIRANGURA, A., GREENBERG, F., BUTLER, M.G., MAL-<br />
COLM, S., NICHOLLS, R.D., CHAKRAVARTI, A., <strong>and</strong> LED-<br />
BETTER, D.H. (1993). Multiplex PCR of three dinucleotide repeats<br />
in the <strong>Prader</strong>-<strong>Willi</strong>/Angelman critical region (15q11–13): molecular<br />
diagnosis <strong>and</strong> mechanism of uniparental disomy. Hum. Mol. Genet.<br />
2, 143–151.<br />
NICHOLLS, R.D., KNOLL, J.H.M., BUTLER, M.G., KARAM, S.,<br />
<strong>and</strong> LALANDE, M. (1989). <strong>Genetic</strong> imprinting suggested by maternal<br />
heterodisomy in non-deletion <strong>Prader</strong>-<strong>Willi</strong> syndrome. Nature<br />
342, 281–285.<br />
OHTA, T., GRAY, T.A., ROGAN, P.K., BUITING, K., GABRIEL,<br />
J.M., SAITOH, S., MURALIGHAR, B., BILENSKA, B., KRA-<br />
JEWSKA-WALASEK, M., DRISCOLL, D.J., HORSTHEMKE, B.,<br />
BUTLER, M.G., <strong>and</strong> NICHOLLS, R.D. (1999). Imprinting-mutation<br />
mechanisms in <strong>Prader</strong>-<strong>Willi</strong> <strong>Syndrome</strong>. Am. J. Hum. Genet. 64,<br />
397–413.<br />
PRADER, A., LABHART, A., <strong>and</strong> WILLI, H. (1956). Ein syndrom<br />
von Adipositas, kleinwuchs, kryptochismus und ologophrenie nach<br />
myotonieartigem zust<strong>and</strong> in neugeborenalter. Schweiz Med.<br />
Wochenschr. 86, 1260–1261.<br />
ROBINSON, W.P., BOTTANI, A., YAGANG, X., BALAKRISH-<br />
MAN, J., BINKERT, F., MACHLER, M., PRADER, A., <strong>and</strong><br />
SCHINZEL, A. (1991). Molecular cytogenetic, <strong>and</strong> clinical investigations<br />
of <strong>Prader</strong>-<strong>Willi</strong> syndrome patients. Am. J. Hum. Genet. 49,<br />
1219–1234.<br />
ROBINSON, W.P., BERNASCONI, F., MUTIRANGURA, A., LED-<br />
BETTER, D.H., LANGLOIS, S., MALCOLM, S., MORRIS, M.A.,<br />
<strong>and</strong> SCHINZEL, A.A. (1993). Nondisjunction of chromosome 15:<br />
origin <strong>and</strong> recombination. Am. J. Hum. Genet. 53, 740–751.<br />
ROBINSON, W.P., KUCHINKA, B.D., BERNASCONI, F., PE-<br />
TERSEN, M.B., SCHULZE, A., BRøNDUM-NIELSEN, K.,<br />
CHRISTIAN, S.L., LEDBETTER, D.H., SCHINZEL, A.A., HORS-<br />
THEMKE, B., SCHUFFENHAUER, S., MICHAELIS, R.C., LAN-<br />
GLOIS, S., <strong>and</strong> HASSOLD, T.J. (1998). Maternal meiosis I non-disjunction<br />
of chromosome 15: dependence of the maternal age effect<br />
on level of recombination. Hum. Mol. Genet. 7, 1011–1019.
392<br />
SAITOH, S., BUITING, K., ROGAN, P.K., BUXTON, J.L.,<br />
DRISCOLL, D.J., ARNEMANN, J., KONIG, R., MALCOLM, S.,<br />
HORSTHEMKE, B., <strong>and</strong> NICHOLLS, R.D. (1996). Minimal definition<br />
of the imprinting center <strong>and</strong> fixation of a chromosome 15q11q13<br />
epigenotype by imprinting mutations. Proc. Natl. Acad. Sci. USA<br />
93, 7811–7815.<br />
SAITOH, S., BUITING, K., CASSIDY, S.B., CONROY, J.M.,<br />
DRISCOLL, D.J., GABRIEL, J.M., GILLESSEN-KAESBACH, G.,<br />
GLENN, C.C., GREENSWAG, L.R., HORSTHEMKE, B.,<br />
KONDO, I., KUWAJIMA, K., NIIKAWA, N., ROGAN, P.K.,<br />
SCHWARTZ, S., SEIP, J., WILLIAMS, C.A., <strong>and</strong> NICHOLLS, R.D.<br />
(1997). <strong>Clinical</strong> spectrum <strong>and</strong> molecular diagnosis of Angelman <strong>and</strong><br />
<strong>Prader</strong>-<strong>Willi</strong> syndrome imprinting mutation patients. Am. J. Med.<br />
Genet. 68, 195–206.<br />
SOUTHERN, E.M. (1975). Detection of specific sequences among<br />
DNA fragments separated by gel eletroforesis. J. Mol. Biol. 98,<br />
503–517.<br />
SPRITZ, R.A., BAILIN, T., NICHOLLS, R.D., LEE, S-T., PARK,<br />
S-K., MASCARI, M.J., <strong>and</strong> BUTLER, M.G. (1997). Hypopigmentation<br />
in the <strong>Prader</strong>-<strong>Willi</strong> syndrome correlates with P gene deletion<br />
but not with haplotype of the hemizygous P allele. Am. J. Med.<br />
Genet. 71, 57–62.<br />
STEIN, C.K., STRED, S.E., THOMSON, L.L., SMITH, F.C., <strong>and</strong><br />
HOO, J.J. (1996). Interstitial 6q deletion <strong>and</strong> <strong>Prader</strong>-<strong>Willi</strong>-like phenotype.<br />
Clin. Genet. 49, 306–310.<br />
STRATAKIS, C.A. (1998). <strong>Prader</strong>-<strong>Willi</strong> syndrome phenotype in X<br />
chromosome anomalies: evidence for a distinct syndrome. Am. J.<br />
Med. Genet. 80, 294–295.<br />
FRIDMAN ET AL.<br />
TÜMER, Z., WOLFF, D., SILAHTAROGLU, A.N., ORUM, A., <strong>and</strong><br />
BRONDUM-NIELSEN, K. (1998). Characterization of a supernumerary<br />
small marker X chromosome in two females with similar phenotypes.<br />
Am. J. Med. Genet. 76, 45–50.<br />
VILLA, A., URIOSTE, M., BOFARULL, J.M., <strong>and</strong> MARTINEZ-<br />
FRIAS, M-L. (1995). De novo interstitial deletion q16.2q21 on chromosome<br />
6. Am. J. Med. Genet. 55, 379–383.<br />
WATRIN, F., ROËCKEL, N., LACROIX, L., MIGNON, C., MAT-<br />
TEI, M-G., DISTECHE, C., <strong>and</strong> MUSCATELLI, F. (1997). The<br />
mouse Necdin gene is expressed from the paternal allele only <strong>and</strong><br />
lies in the 7C region of the mouse chromosome 7, a region conserved<br />
synteny to the human <strong>Prader</strong>-<strong>Willi</strong> syndrome region. Eur. J. Hum.<br />
Genet. 5, 324–332.<br />
Address reprint requests to:<br />
Dr. C. Fridman<br />
Departamento de Biologia<br />
Instituto de Biociências, <strong>USP</strong><br />
Caixa Postal 11.461<br />
CEP: 05422-970, São Paulo, SP, Brazil<br />
E-mail: cfridman@ib.usp.br<br />
Received for publication December 2, 1999; accepted July 7,<br />
2000.