DISSERTATION
resolver
resolver
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
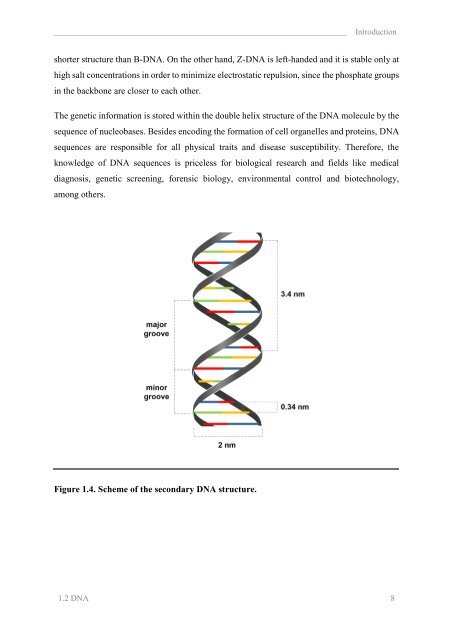
______________________________________________________________________ Introduction<br />
1.2.1 DNA in solution<br />
DNA is a highly charged polymer with charge density equal to two elementary charges per base<br />
pair. Therefore, in solution DNA interacts strongly with surrounding ions that counterbalance<br />
its charge. These ions can be grouped into different zones 31 . In the region closest to the DNA<br />
there are so called site-bound or inner-sphere ions that share water molecules with the DNA. In<br />
the following zone are outer-sphere ions (territorial ions) that keep their inner hydration layer<br />
and are free to move along the DNA molecule but are kept close to it due to the electrostatic<br />
field. And in the last zone are free ions that form an ionic cloud around the DNA 3 .<br />
The two most known approaches employed to investigate the extent of DNA-ion interaction are<br />
the Manning-Oosawa (MO) counterion condensation theory 32 and the Poisson-Boltzmann (PB)<br />
equation. MO theory addresses the DNA charge compensation by focusing on the counterions<br />
that form a so-called condensed layer around the DNA (outer-sphere ions) in order to reduce<br />
the charge density below a certain critical value 33 . According to the theory, counterion<br />
accumulation at the DNA surface forming a condensed layer occurs under the condition that<br />
the charge density parameter η is > 1:<br />
η = zl B<br />
b<br />
(1.4)<br />
where z is the valence of counterions, lB is the Bjerrum length and b is the charge separation.<br />
The Bjerrum length represents the distance between charges at which their electrostatic<br />
interaction energy equals the thermal energy and is defined as:<br />
l B =<br />
e 2<br />
4πεε 0 kT<br />
(1.5)<br />
where e is the elementary charge, ε is the dielectric constant of the solvent, ε0 is the vacuum<br />
permeability and kT is thermal energy scale. This means that the counterion condensation (for<br />
monovalent ions) occurs when the charge separation b is smaller than the Bjerrum length, which<br />
is true for both ss- and dsDNA. Namely, lB = 0.71 nm in aqueous solutions, while the charge<br />
separation is 0.43 nm in ssDNA and 0.34 nm in dsDNA 31 . According to the theory, condensed<br />
counterions are still assumed to be mobile 34 .<br />
1.2 DNA 9