IPP Annual Report 2007 - Max-Planck-Institut für Plasmaphysik ...
IPP Annual Report 2007 - Max-Planck-Institut für Plasmaphysik ...
IPP Annual Report 2007 - Max-Planck-Institut für Plasmaphysik ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Charged Water Clusters<br />
Many aspects of the photoelectron<br />
spectra of free molecules<br />
are well understood. On the other<br />
hand, electron spectroscopy of<br />
liquids has only become possible<br />
recently and is a field of rapidly<br />
growing interest. Photoelectron<br />
spectra of liquid water differ in a<br />
number of respects from the mole-<br />
cule: Features due to ionic state vibrations, a characteristic<br />
of molecular conformational changes, are smeared out or<br />
absent in the liquid. Ionization energies of all orbitals shift towards<br />
lower values. The value of the vertical ionization energy<br />
of the least strongly bound electrons in the liquid has been<br />
measured some years ago. In our study of free water clusters,<br />
we show how this literature value is smoothly approached<br />
when ionization of clusters of increasing size is studied.<br />
HOMO Energy Shift to Monomer (eV)<br />
1.6<br />
1.4<br />
1.2<br />
1.0<br />
0.8<br />
0.6<br />
0.4<br />
0.2<br />
0.0<br />
0.0<br />
0.2<br />
0.4 0.6<br />
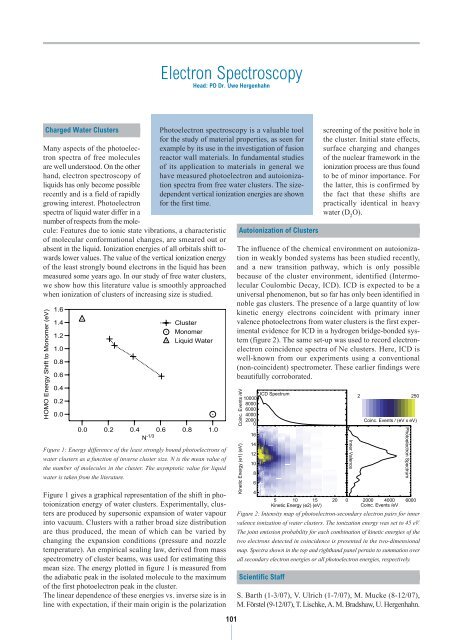
N-1/3 Figure 1 gives a graphical representation of the shift in photoionization<br />
energy of water clusters. Experimentally, clusters<br />
are produced by supersonic expansion of water vapour<br />
into vacuum. Clusters with a rather broad size distribution<br />
are thus produced, the mean of which can be varied by<br />
changing the expansion conditions (pressure and nozzle<br />
temperature). An empirical scaling law, derived from mass<br />
spectrometry of cluster beams, was used for estimating this<br />
mean size. The energy plotted in figure 1 is measured from<br />
the adiabatic peak in the isolated molecule to the maximum<br />
of the first photoelectron peak in the cluster.<br />
The linear dependence of these energies vs. inverse size is in<br />
line with expectation, if their main origin is the polarization<br />
Electron Spectroscopy<br />
Head: PD Dr. Uwe Hergenhahn<br />
Photoelectron spectroscopy is a valuable tool<br />
for the study of material properties, as seen for<br />
example by its use in the investigation of fusion<br />
reactor wall materials. In fundamental studies<br />
of its application to materials in general we<br />
have measured photoelectron and autoionization<br />
spectra from free water clusters. The sizedependent<br />
vertical ionization energies are shown<br />
for the first time.<br />
Cluster<br />
Monomer<br />
Liquid Water<br />
0.8<br />
1.0<br />
Figure 1: Energy difference of the least strongly bound photoelectrons of<br />
water clusters as a function of inverse cluster size. N is the mean value of<br />
the number of molecules in the cluster. The asymptotic value for liquid<br />
water is taken from the literature.<br />
101<br />
Autoionization of Clusters<br />
screening of the positive hole in<br />
the cluster. Initial state effects,<br />
surface charging and changes<br />
of the nuclear framework in the<br />
ionization process are thus found<br />
to be of minor importance. For<br />
the latter, this is confirmed by<br />
the fact that these shifts are<br />
practically identical in heavy<br />
water (D 2 O).<br />
The influence of the chemical environment on autoionization<br />
in weakly bonded systems has been studied recently,<br />
and a new transition pathway, which is only possible<br />
because of the cluster environment, identified (Intermolecular<br />
Coulombic Decay, ICD). ICD is expected to be a<br />
universal phenomenon, but so far has only been identified in<br />
noble gas clusters. The presence of a large quantity of low<br />
kinetic energy electrons coincident with primary inner<br />
valence photoelectrons from water clusters is the first experimental<br />
evidence for ICD in a hydrogen bridge-bonded system<br />
(figure 2). The same set-up was used to record electronelectron<br />
coincidence spectra of Ne clusters. Here, ICD is<br />
well-known from our experiments using a conventional<br />
(non-coincident) spectrometer. These earlier findings were<br />
beautifully corroborated.<br />
Coinc. Events /eV<br />
Kinetic Energy (e1) (eV)<br />
10000<br />
8000<br />
6000<br />
4000<br />
2000<br />
0<br />
16<br />
14<br />
12<br />
10<br />
8<br />
6<br />
4<br />
ICD Spectrum<br />
Scientific Staff<br />
5 10 15 20<br />
Kinetic Energy (e2) (eV)<br />
250<br />
S. Barth (1-3/07), V. Ulrich (1-7/07), M. Mucke (8-12/07),<br />
M. Förstel (9-12/07), T. Lischke, A. M. Bradshaw, U. Hergenhahn.<br />
Inner Valence<br />
2<br />
Coinc. Events / (eV x eV)<br />
Photoelectron Spectrum<br />
0 2000 4000 6000<br />
Coinc. Events /eV<br />
Figure 2: Intensity map of photoelectron-secondary electron pairs for inner<br />
valence ionization of water clusters. The ionization energy was set to 45 eV.<br />
The joint emission probability for each combination of kinetic energies of the<br />
two electrons detected in coincidence is presented in the two-dimensional<br />
map. Spectra shown in the top and righthand panel pertain to summation over<br />
all secondary electron energies or all photoelectron energies, respectively.