Partial Periodic Table - Organic Chemistry at CU Boulder ...
Partial Periodic Table - Organic Chemistry at CU Boulder ...
Partial Periodic Table - Organic Chemistry at CU Boulder ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
CHEMISTRY 3311, Fall 2003<br />
Professor Walba<br />
Third Hour Exam, November 20<br />
scores:<br />
1) 20<br />
2) 20<br />
3) 20<br />
4) 30<br />
5) 10<br />
100<br />
<strong>CU</strong> Honor Code Pledge: On my honor, as a University of Colorado <strong>at</strong><br />
<strong>Boulder</strong> Student, I have neither given nor received unauthorized assistance.<br />
Name (printed): _________________________________<br />
Key<br />
Sign<strong>at</strong>ure: ______________________________________<br />
Recit<strong>at</strong>ion TA Name: _____________________________<br />
Recit<strong>at</strong>ion day and time: ___________________________<br />
This is a closed-book exam. The use of notes, models,<br />
calcul<strong>at</strong>ors, and other paraphernalia will not be allowed during the<br />
exam. Please put all your answers on the test. Use the backs of<br />
the pages for scr<strong>at</strong>ch.<br />
PLEASE read the questions carefully!<br />
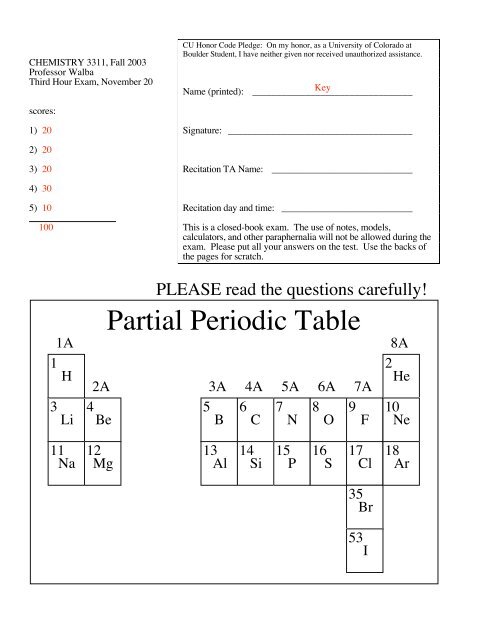
<strong>Partial</strong> <strong>Periodic</strong> <strong>Table</strong><br />
1A<br />
8A<br />
1<br />
H<br />
2A 3A 4A 5A 6A 7A<br />
2<br />
He<br />
3<br />
Li<br />
4<br />
Be<br />
5<br />
B<br />
6<br />
C<br />
7<br />
N<br />
8<br />
O<br />
9<br />
F<br />
10<br />
Ne<br />
11 12 13 14 15 16 17 18<br />
Na Mg Al Si P S Cl Ar<br />
35<br />
Br<br />
53<br />
I
Name:<br />
1) (20 pts) a) Arrange the following three anions in order of decreasing Bronsted bacisity.<br />
NH 2<br />
-2- 2<br />
CH 3CH 2<br />
1 2 3<br />
Strongest<br />
base<br />
H<br />
C C<br />
> ><br />
3 1<br />
2<br />
Weakest<br />
base<br />
b) The reaction of cyclopentadiene (1) with maleic anhydride (2) is one of the most famous Diels Alder reactions.<br />
Two stereoisomeric products are formed, 3 and 4, named the endo and exo products, respectively.<br />
+<br />
1 2<br />
O<br />
O<br />
O<br />
he<strong>at</strong><br />
O<br />
O<br />
O<br />
3 (endo) 4 (exo)<br />
Given the following inform<strong>at</strong>ion, complete the energy diagram, carefully indic<strong>at</strong>ing the structure of each “well”<br />
using the numbers (3 and 4). There is no need to label the transition st<strong>at</strong>es, but be sure to complete the diagram in<br />
such a way th<strong>at</strong> it’s clear which product came from which transition st<strong>at</strong>e.<br />
1. Both products 3 and 4 are more stable than the starting m<strong>at</strong>erials.<br />
2. At low temper<strong>at</strong>ure the reaction is irreversible (kinetic conditions), and product 3 is the major product.<br />
3 At high temper<strong>at</strong>ure the reaction is reversible (thermodynamic conditions), and product 4 is the major product.<br />
E<br />
1 + 2<br />
3<br />
H<br />
H<br />
Reaction Coordin<strong>at</strong>e<br />
+<br />
4<br />
H H<br />
O<br />
O<br />
O
Name:<br />
2) (20 pts) Give the single major organic product of each of the following reactions. Carefully indic<strong>at</strong>e the<br />
stereochemistry of the product if appropri<strong>at</strong>e. If a racem<strong>at</strong>e is formed, show only one enantiomer, and label it<br />
“rac.”<br />
a)<br />
b)<br />
c)<br />
d)<br />
Br<br />
Br<br />
CH 3 CH 2 O<br />
CH 3 CH 2 O<br />
Br NaSCH 2 CH 3<br />
O , ∆<br />
-3- 3<br />
rac<br />
rac<br />
S<br />
O<br />
O
Name:<br />
3) (20 pts) Propose reagents for accomplishing the following transform<strong>at</strong>ions. NOTE: more than one step may<br />
be required! Try to make your synthesis efficient (i.e. the desired product should be the major product). You must<br />
use the starting m<strong>at</strong>erial given; you may use any other reagents you need.<br />
a)<br />
b)<br />
c)<br />
d)<br />
H 3 CO Br H 3 CO CN<br />
H H<br />
NaCN, DMSO<br />
NBS, CCl 4<br />
∆<br />
1) a) NaNH 2<br />
b) CH3CH2Br<br />
2) H 2 SO 4 , H 2 O, HgSO 4<br />
OH 1) TsCl, pyr<br />
S<br />
2) CH 3 CH 2 CH 2 SNa<br />
-4- 4<br />
O<br />
Br
Name:<br />
4) (30 pts) When the bromide 1 is dissolved in methanol, the ethers 2 and 3 are formed. It can be shown th<strong>at</strong> the<br />
reaction is irreversible.<br />
a) Wh<strong>at</strong> is the name of the mechanism for this transform<strong>at</strong>ion? S N1<br />
b) Circle the major product of this reaction.<br />
c) Propose and arrow-pushing mechanism for the form<strong>at</strong>ion of ethers 2 and 3. In your mechanism, be sure to<br />
show each intermedi<strong>at</strong>e using valid valence-bond formulas.<br />
Br<br />
+<br />
CH3OH +<br />
1<br />
Br<br />
2<br />
OCH3 H3CO 3<br />
AND<br />
HOCH 3<br />
Br<br />
HOCH 3<br />
-5- 5<br />
O<br />
H<br />
O<br />
H<br />
H 3CO<br />
OCH 3
4 –continued-<br />
Name:<br />
d) When the bromide 4 is dissolved in methanol, a new product is formed in high yield. This product has no<br />
OCH 3 groups, and only one double bond (!). The molecular formula of the mystery product is C 6 H 10 O. For five<br />
bonus points, give the structure of the product of this transform<strong>at</strong>ion.<br />
e) Propose an arrow-pushing mechanism for the chain propag<strong>at</strong>ion steps for following transform<strong>at</strong>ion. Please<br />
show only the chain propag<strong>at</strong>ion steps in your mechanism.<br />
Br<br />
HO CH 3OH<br />
H<br />
Br<br />
4 C 6 H 10 O<br />
H HBr, peroxides<br />
Br<br />
H<br />
Br<br />
-6- 6<br />
O<br />
Br<br />
Br<br />
Br
Name:<br />
5) (10 pts) Propose a synthesis for the following target using any organic starting m<strong>at</strong>erials with FIVE carbons or<br />
less, and any inorganic reagents you need. Try to make your synthesis efficient (th<strong>at</strong> is, the desired product in each<br />
step should be the major product).<br />
Na, Liq. NH 3<br />
-7- 7<br />
a) NaNH 2<br />
b) Br<br />
H<br />
a) NaNH 2<br />
b) Br<br />
H H