Protocol Synopsis V1.1 - Rare Disease
Protocol Synopsis V1.1 - Rare Disease
Protocol Synopsis V1.1 - Rare Disease
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
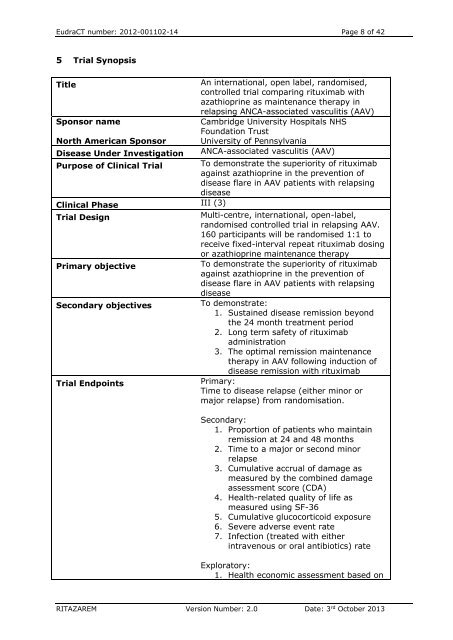
EudraCT number: 2012-001102-14 Page 8 of 425 Trial <strong>Synopsis</strong>TitleSponsor nameNorth American Sponsor<strong>Disease</strong> Under InvestigationAn international, open label, randomised,controlled trial comparing rituximab withazathioprine as maintenance therapy inrelapsing ANCA-associated vasculitis (AAV)Cambridge University Hospitals NHSFoundation TrustUniversity of PennsylvaniaANCA-associated vasculitis (AAV)Purpose of Clinical Trial To demonstrate the superiority of rituximabagainst azathioprine in the prevention ofdisease flare in AAV patients with relapsingdiseaseClinical Phase III (3)Trial DesignMulti-centre, international, open-label,randomised controlled trial in relapsing AAV.160 participants will be randomised 1:1 toreceive fixed-interval repeat rituximab dosingor azathioprine maintenance therapyPrimary objectiveTo demonstrate the superiority of rituximabagainst azathioprine in the prevention ofdisease flare in AAV patients with relapsingdiseaseSecondary objectivesTo demonstrate:1. Sustained disease remission beyondthe 24 month treatment period2. Long term safety of rituximabadministration3. The optimal remission maintenancetherapy in AAV following induction ofdisease remission with rituximabTrial EndpointsPrimary:Time to disease relapse (either minor ormajor relapse) from randomisation.Secondary:1. Proportion of patients who maintainremission at 24 and 48 months2. Time to a major or second minorrelapse3. Cumulative accrual of damage asmeasured by the combined damageassessment score (CDA)4. Health-related quality of life asmeasured using SF-365. Cumulative glucocorticoid exposure6. Severe adverse event rate7. Infection (treated with eitherintravenous or oral antibiotics) rateExploratory:1. Health economic assessment based onRITAZAREM Version Number: 2.0 Date: 3 rd October 2013
EudraCT number: 2012-001102-14 Page 9 of 42EQ5D2. Health-related quality of life andpatient-reported domains as measuredusing PROMIS3. Serum rituximab levels, andcorrelation with circulating B cellcounts including key subsets andimmunoglobulin levels4. Changes in ANCA titres (both anti-MPOand anti-PR3 subsets) in relation totreatment, response, and relapse.5. HACA rate and levels6. Serum will be stored for futurebiomarker studies7. mRNA will stored for disease andinflammatory gene activation studies8. DNA will be stored for future geneticstudiesSample SizeSummary of Eligibility CriteriaEnrolment will be ongoing until 160 patientsare randomised. It is anticipated this willrequire 190 patients to be recruited.Subjects must meet all of the followingcriteria to be eligible for enrolment:1. Written informed consent (15 yearsand above)2. A diagnosis of AAV (granulomatosiswith polyangiitis (Wegener's) ormicroscopic polyangiitis), according tothe definitions of the Chapel HillConsensus Conference3. Current or historical PR3/MPO ANCApositivity by ELISA4. <strong>Disease</strong> relapse defined by one majoror three minor disease activity itemson the Birmingham Vasculitis ActivityScore for Wegener’s (BVAS/WG), inpatients that have previously achievedremission following induction therapyKey Exclusion CriteriaInvestigational MedicinalProduct and Dosage1. Age < 15 years (age < 18 years atcentres that do not treat paediatricpatients)2. Previous therapy with any biological B celldepleting agent (such as rituximab orbelimumab) within the past 6 monthsRituximabInduction RegimenPatients will be recruited at the time ofrelapse. All will receive rituximab375 mg/m 2 /week x 4 doses andRITAZAREM Version Number: 2.0 Date: 3 rd October 2013
EudraCT number: 2012-001102-14 Page 10 of 42glucocorticoids.Patients that achieve disease control(BVAS/WG ≤ 1 and daily prednisone dose ≤10mg) by month 4 will be randomised to therituximab or control remission maintenancegroups.Rituximab maintenance groupRituximab 1000 mg at months 4, 8, 12, 16and 20 and glucocorticoids.Active Comparator ProductsAzathioprineInduction RegimenPatients will be recruited at the time ofrelapse. All will receive rituximab375 mg/m 2 /week x 4 and glucocorticoids.Those patients that achieve disease control(BVAS/WG ≤ 1 and daily prednisone dose≤ 10 mg) by month 4 will be randomised tothe rituximab or control remissionmaintenance groups.Route of AdministrationConcomitant TherapyControl maintenance groupAzathioprine 2 mg/kg/day from month 4 to24. Dose reductions for intolerance, abnormalliver function tests, cytopenias, or based onabnormal TPMT genotype/activity testing (ifperformed) will be made. Methotrexate25 mg/week, for patients with GFR >50 ml/min and intolerant of azathioprine evenat a reduced dose of 1 mg/kg/day, ormycophenolate mofetil 2 g/day, for patientsintolerant of azathioprine and with GFR
EudraCT number: 2012-001102-14 Page 11 of 42permitted GC regimens:Oral prednisone (prednisolone will beallowed), commencing at 1.0mg/kg/day(maximum 60 mg/day),or 0.5mg/kg/day (maximum 30 mg/day),both reducing to 10mg/day by month 3 (Seeschedule 1, pg. 26).Maximum daily dose of GC is 60 mgprednisone in week 0. Round down to thenearest 5 mg above 20 mg. Round down tothe nearest 2.5 mg below 20 mg. GC to beadministered in a single daily dose.Intravenous (IV) GCs are not mandated bythe protocol. At the investigator’s discretion,patients may receive up to a maximumcumulative dose of 3000mg IVmethylprednisolone, between 14 days prior toenrolment and 7 days after enrolment.Glucocorticoids in Maintenance Phase:Maximum Treatment DurationSummary of Study ProceduresScreening:Baseline:A GC dose of prednisolone 10 mg/day or lessis a requirement for randomisation. GCreduces to 5 mg/day by month 6, accordingto schedule 2 (pg. 26). At month 16, GCdose is reduced to 2.5mg/day and at month20 GC are completely withdrawn.Treatment is protocolised for the entireduration of the study, until the common closedate, when the final patient recruited hascompleted 36 months within the study oruntil the patient has completed 48 months onstudy whichever the sooner. Patients in therituximab arm will receive treatment untilmonth 20, and those in the azathioprine armuntil month 27.Procedures to establish inclusion/exclusioncriteria.Baseline data will include:1. Date of birth, sex, limited medicalhistory2. Medications, including prior AAVtreatments3. Height/weight4. <strong>Disease</strong> activity assessment(BVAS/WG)5. <strong>Disease</strong> related damage assessment(CDA)6. Patient self-reported SF-36questionnaire, EQ5D questionnaire andRITAZAREM Version Number: 2.0 Date: 3 rd October 2013
EudraCT number: 2012-001102-14 Page 12 of 42PROMIS questionnaires.Treatment period: Evaluations will be performed at months 0,1.5, 3, 4, 8, 12, 16, 20, 24, 27, 30, 36, andevery 6 months until the last patient hascompleted 36 months in the study.The maximum duration in the study is 48months. Assessments will also be performedat the time of relapse or study termination/withdrawal.End of Trial:The trial will end when the last patient hascompleted 36 months in the trial (fromenrolment).Safety and MonitoringThe NIH-sponsored VCRC Data and SafetyMonitoring Board will provide independentoversight of this trial.RITAZAREM Version Number: 2.0 Date: 3 rd October 2013