Organic Chemistry
Chirality
Chirality
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
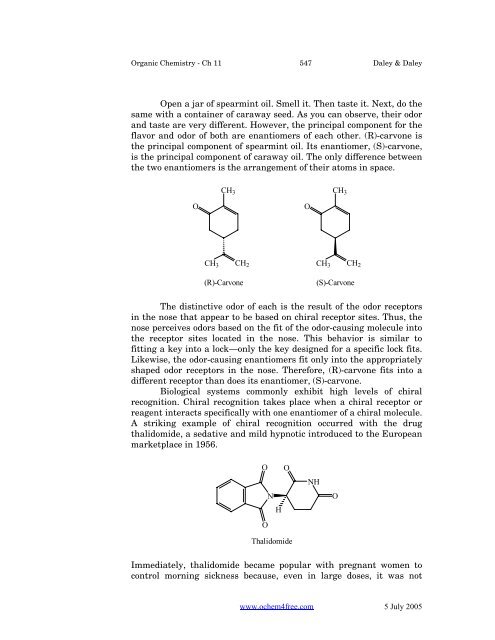
<strong>Organic</strong> <strong>Chemistry</strong> - Ch 11 547 Daley & DaleyOpen a jar of spearmint oil. Smell it. Then taste it. Next, do thesame with a container of caraway seed. As you can observe, their odorand taste are very different. However, the principal component for theflavor and odor of both are enantiomers of each other. (R)-carvone isthe principal component of spearmint oil. Its enantiomer, (S)-carvone,is the principal component of caraway oil. The only difference betweenthe two enantiomers is the arrangement of their atoms in space.CH 3CH 2CH 3CH 2OOCH 3(R)-CarvoneCH 3(S)-CarvoneThe distinctive odor of each is the result of the odor receptorsin the nose that appear to be based on chiral receptor sites. Thus, thenose perceives odors based on the fit of the odor-causing molecule intothe receptor sites located in the nose. This behavior is similar tofitting a key into a lock—only the key designed for a specific lock fits.Likewise, the odor-causing enantiomers fit only into the appropriatelyshaped odor receptors in the nose. Therefore, (R)-carvone fits into adifferent receptor than does its enantiomer, (S)-carvone.Biological systems commonly exhibit high levels of chiralrecognition. Chiral recognition takes place when a chiral receptor orreagent interacts specifically with one enantiomer of a chiral molecule.A striking example of chiral recognition occurred with the drugthalidomide, a sedative and mild hypnotic introduced to the Europeanmarketplace in 1956.O ONHOThalidomideNHOImmediately, thalidomide became popular with pregnant women tocontrol morning sickness because, even in large doses, it was notwww.ochem4free.com 5 July 2005