Land Contamination: Technical Guidance on Special Sites: Acid Tar ...
Land Contamination: Technical Guidance on Special Sites: Acid Tar ... Land Contamination: Technical Guidance on Special Sites: Acid Tar ...
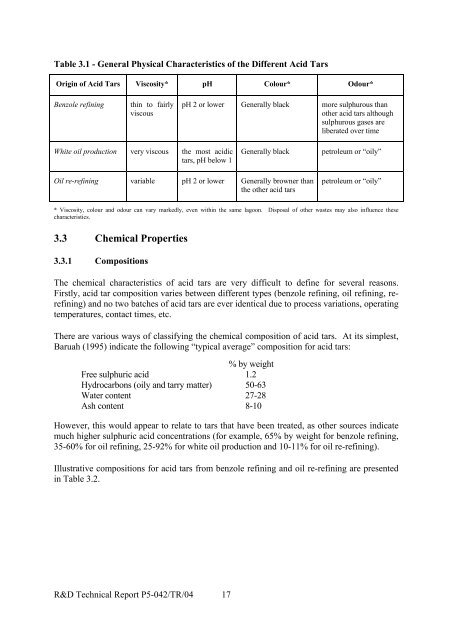
3.2.2 pHThe pH of acid tars is variable and dependent on the following:• type of process that generated the acid tar;• the amount and strength of sulphuric acid used in the process; and• the degree of pre-treatment prior to disposal.White oil refining gives rise to the most acidic tars, being below pH 1. Benzole refining isless severe (as aromatics must not be sulphonated to any significant extent); re-refining isvariable. However, pH values are frequently 2 or lower unless wastes have been pre-treated.As mentioned previously, at some sites the tars may have been neutralised prior to disposal.At others there may have been steam stripping/washing to remove and recover some of thesulphuric acid (for ammonium sulphate manufacture). A further method involved use ofsolvent oil (creosote) to extract sulphuric acid. Typically, however, the tars will have beenplaced in lagoons to “weather”.3.2.3 ColourTars from re-refining (containing some oils) are generally browner than those from white oilproduction and benzole refining, which are darker (essentially black) and thicker. Acid tarsthat are exposed at the ground surface will weather over time and may take on a dry, brownishappearance.3.2.4 OdourAll acid tars are odorous. Re-refining tars are generally characterised by petroleum or "oily"odours, while the benzole tars are generally more sulphurous. Sulphurous odours tend todisappear with time as sulphur dioxide is released from the tars. Other sulphur-basedcompounds, including thiophenes and mercaptans, may volatilise from the lagoon surface andthese can be odorous. Hydrogen sulphide may also be released at low levels. The weatheringprocess results in little odour or air pollution from the lagoon, although investigation andremediation will disturb the lower layers and may give rise to acidic vapours and the releaseof more volatile hydrocarbons.The general physical properties of the different types of acid tars are summarised in Table 3.1,although it should be noted that colour, odour and viscosity can vary markedly, even withinthe same lagoon.R&D
Table 3.1 - General Physical Characteristics of the Different Acid TarsOrigin of Acid Tars Viscosity* pH Colour* Odour*Benzole refiningthin to fairlyviscouspH 2 or lower Generally black more sulphurous thanother acid tars althoughsulphurous gases areliberated over timeWhite oil production very viscous the most acidictars, pH below 1Generally blackpetroleum or “oily”Oil re-refining variable pH 2 or lower Generally browner thanthe other acid tarspetroleum or “oily”* Viscosity, colour and odour can vary markedly, even within the same lagoon. Disposal of other wastes may also influence thesecharacteristics.3.3 Chemical Properties3.3.1 CompositionsThe chemical characteristics of acid tars are very difficult to define for several reasons.Firstly, acid tar composition varies between different types (benzole refining, oil refining, rerefining)and no two batches of acid tars are ever identical due to process variations, operatingtemperatures, contact times, etc.There are various ways of classifying the chemical composition of acid tars. At its simplest,Baruah (1995) indicate the following “typical average” composition for acid tars:% by weightFree sulphuric acid 1.2Hydrocarbons (oily and tarry matter) 50-63Water content 27-28Ash content 8-10However, this would appear to relate to tars that have been treated, as other sources indicatemuch higher sulphuric acid concentrations (for example, 65% by weight for benzole refining,35-60% for oil refining, 25-92% for white oil production and 10-11% for oil re-refining).Illustrative compositions for acid tars from benzole refining and oil re-refining are presentedin Table 3.2.R&D
- Page 1 and 2: Land Conta
- Page 3 and 4: CONTENTSFOREWORDiGLOSSARYii1. Intro
- Page 5 and 6: FOREWORDPart IIA of the Environment
- Page 7 and 8: CresolsCreosoteDCRDesorptionDesulph
- Page 9 and 10: PVCRCRASelosafeSodium saltsSodium h
- Page 11 and 12: 1. INTRODUCTION1.1 BackgroundThis r
- Page 13 and 14: SECTION 2: INDUSTRIAL PROCESS/FACIL
- Page 15 and 16: 1.5 Linkages Between the Gu
- Page 17 and 18: 2.1.2 Overview of acid tar lagoon s
- Page 19 and 20: Overall CommentsAcid tars can be of
- Page 21 and 22: White Oil• Acid tar lagoons from
- Page 23 and 24: Box 2.5 Scenario: Acid tar disposal
- Page 25: 3. CHEMICAL AND PHYSICAL CHARACTERI
- Page 29 and 30: The chemicals listed in Table B.1,
- Page 31 and 32: 4.2.3 Common mistakesIt is possible
- Page 33 and 34: Geophysical methods, such as resist
- Page 35 and 36: Investigation locations will need t
- Page 37 and 38: • gas chromatography by simulatin
- Page 39 and 40: 5.2.2 Water environmentThere is lit
- Page 41 and 42: 5.3 Assessment of Investigation Dat
- Page 43 and 44: 6. REMEDIATION ASPECTSKEY QUESTIONS
- Page 45 and 46: 6.2.5 Solidification and stabilisat
- Page 47 and 48: Box 6.1 Example of Remediation of A
- Page 49 and 50: 6.3 Natural AttenuationNatural atte
- Page 51 and 52: Table 6.1 - Remedial Options/Techni
- Page 53 and 54: Table 6.2 - Remedial Options/Techni
- Page 55 and 56: 6.4 ValidationThe principles of val
- Page 57 and 58: • all personnel should follow a d
- Page 59 and 60: Verschueren (1983) Handbook of Envi
- Page 61 and 62: USEPA. Innovative Site Remediation
Table 3.1 - General Physical Characteristics of the Different <strong>Acid</strong> <strong>Tar</strong>sOrigin of <strong>Acid</strong> <strong>Tar</strong>s Viscosity* pH Colour* Odour*Benzole refiningthin to fairlyviscouspH 2 or lower Generally black more sulphurous thanother acid tars althoughsulphurous gases areliberated over timeWhite oil producti<strong>on</strong> very viscous the most acidictars, pH below 1Generally blackpetroleum or “oily”Oil re-refining variable pH 2 or lower Generally browner thanthe other acid tarspetroleum or “oily”* Viscosity, colour and odour can vary markedly, even within the same lago<strong>on</strong>. Disposal of other wastes may also influence thesecharacteristics.3.3 Chemical Properties3.3.1 Compositi<strong>on</strong>sThe chemical characteristics of acid tars are very difficult to define for several reas<strong>on</strong>s.Firstly, acid tar compositi<strong>on</strong> varies between different types (benzole refining, oil refining, rerefining)and no two batches of acid tars are ever identical due to process variati<strong>on</strong>s, operatingtemperatures, c<strong>on</strong>tact times, etc.There are various ways of classifying the chemical compositi<strong>on</strong> of acid tars. At its simplest,Baruah (1995) indicate the following “typical average” compositi<strong>on</strong> for acid tars:% by weightFree sulphuric acid 1.2Hydrocarb<strong>on</strong>s (oily and tarry matter) 50-63Water c<strong>on</strong>tent 27-28Ash c<strong>on</strong>tent 8-10However, this would appear to relate to tars that have been treated, as other sources indicatemuch higher sulphuric acid c<strong>on</strong>centrati<strong>on</strong>s (for example, 65% by weight for benzole refining,35-60% for oil refining, 25-92% for white oil producti<strong>on</strong> and 10-11% for oil re-refining).Illustrative compositi<strong>on</strong>s for acid tars from benzole refining and oil re-refining are presentedin Table 3.2.R&D <str<strong>on</strong>g>Technical</str<strong>on</strong>g> Report P5-042/TR/04 17